Conversion from Immediate to Extended-Release Tacrolimus (LCP-Tac) Six Months After Renal Transplantation Optimizes Safety and Efficacy
MUSC Health, Charleston, SC
Meeting: 2022 American Transplant Congress
Abstract number: 1685
Keywords: Infection, Kidney transplantation, Pharmacokinetics, Rejection
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: LCP-Tac is a once daily formulation of tacrolimus indicated for prevention of rejection in kidney transplant recipients (KTRs) that may have less variability and equivalent overall drug exposure. We sought to determine the impact of early conversion (within six months) or late (greater than six months) after transplant from immediate-release tacrolimus (IR-Tac) to LCP-Tac on the safety and efficacy of LCP-Tac in KTRs.
*Methods: This was a retrospective longitudinal cohort study of KTRs converted to LCP-Tac between Jun 2019 and Oct 2020 with follow-up through Nov 2021. Patients were excluded if they were in a clinical trial, had non-renal, non-pancreas transplants, were prescribed LCP-Tac for less than 30 days, lost to follow-up, or died within one month of conversion. The primary efficacy outcome was the incidence of biopsy proven acute rejection. The primary safety outcome was the incidence of total infections. Data was analyzed using chi-squared or 2-sample t-test for categorical and continuous data, respectively.
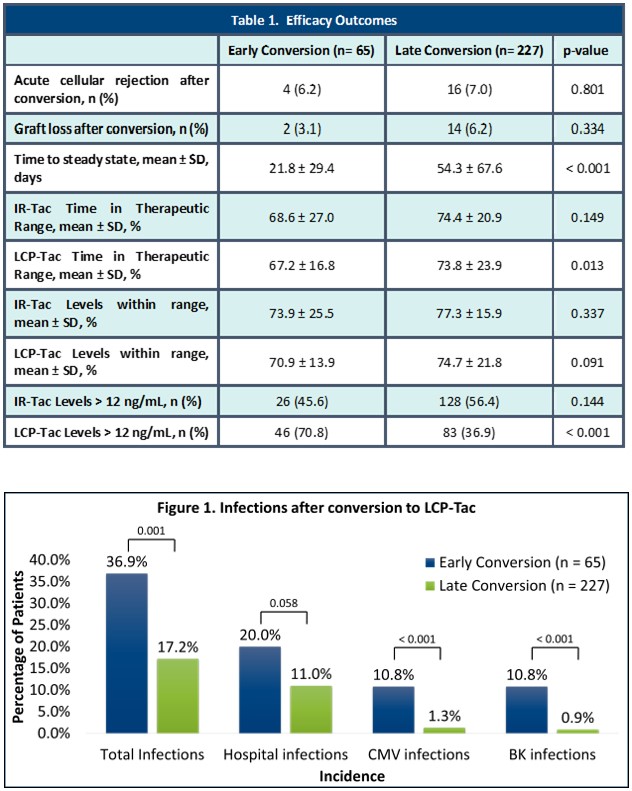
*Results: 337 patients were reviewed and 292 were included. Patients converted to LCP-Tac late post-transplant had a significantly lower incidence of supratherapeutic tacrolimus levels and greater time in therapeutic range than patients converted early post-transplant. Patients who were converted later had a significantly greater time to steady state than patients who were converted early while those converted to LCP-Tac had a significantly higher rate of infection if converted early vs late, primarily due to the increase in CMV and BK infections. There was a significantly higher rate of neurotoxicity in patients who were converted early post-transplant (n=17, 26.2%) than those converted late post-transplant (n=21, 9.3%; p-<0.001). There was no significant difference in the incidence of biopsy proven acute cellular rejection and tacrolimus levels within range in patients converted to LCP-Tac early versus late post-transplant. There was no difference in death or graft loss across groups.
*Conclusions: These results suggest the optimal period for conversion from IR-Tac to LCP-Tac is around six months post-transplant, which offers the best balance of efficacy and safety.
To cite this abstract in AMA style:
Patel SP, Bartlett F, Sprague T, Newman J, Andrade E, Rao N, Patel N, Dubay D, Rohan V, Casey M, Taber D. Conversion from Immediate to Extended-Release Tacrolimus (LCP-Tac) Six Months After Renal Transplantation Optimizes Safety and Efficacy [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-from-immediate-to-extended-release-tacrolimus-lcp-tac-six-months-after-renal-transplantation-optimizes-safety-and-efficacy/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress