Contemporary Analysis of the Effect of Induction Immunosuppression of Survival in Pediatric Lung Transplant Recipients, A
Department of Pediatrics, The Ohio State University, Columbus, OH
Department of Internal Medicine, The Ohio State University, Columbus, OH
Center for Biostatistics, The Ohio State University, Columbus, OH
Department of Surgery, The Ohio State University, Columbus, OH
Meeting: 2013 American Transplant Congress
Abstract number: 247
Introduction:
The role of induction immunosuppression in lung transplantation (LTX) is varied. In the pediatric population, due to a smaller national incidence and center practice variability, the impact is more unclear. We sought to evaluate the effect of contemporary induction immunosuppression in LTX recipients on survival, utilizing national registry data.
Methods:
We queried the United Network for Organ Sharing (UNOS) data registry for all lung transplants from 1987 to 2012, restricting analysis to 6-18 years of age, cadaveric lung transplants performed from 2001-2012 who received either: no induction (NONE) or contemporary agents of basiliximab, alemtuzumab, thymoglobulin, ALG, or ATG (INDUCED). Kaplan-Meier estimates and Cox proportional hazards models were used to compare survival.
Results:
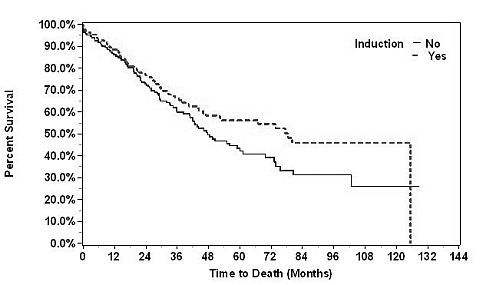
23,951 lung transplants were performed with 400 meeting inclusion criteria, of those 214 (53.5%) were INDUCED. Of the INDUCED agents, 68% were basiliximab, 3% alemtuzumab, and 29% ALG/ATG/Thymo. The indications for transplantation were: 70.7% CF, 9.3% IPF/OB/PF, 8.5% PPH, and 11.5% other. The mean patient age was 14.4 (SD=3.4) for the INDUCED and 14.7 (SD=3.1) for the NONE group. The median survival in the INDUCED group was 78.5 months (95% CI: 46.9-125.4) as compared to 47.6 months (95% CI: 41-61.2) for the NONE (log rank p-value comparing survival curves = 0.063; hazard ratio from Cox model = 0.75 for INDUCED vs. NONE, 95% CI: 0.55-01.02, p=0.064). Of the INDUCED patients, 23% were treated for rejection, compared to 25% for the NONE patients. Note however that 25% of these data were uncoded; multiple imputation will be used to assess the impact of the missing data.
Conclusion:
In a contemporary analysis of pediatric LTX recipients, induction immunosuppression appears to have a positive effect on survival. These data support the development of multicenter prospective trials to further delineate this effect.
To cite this abstract in AMA style:
Hayes D, Kirkby S, Wehr A, Lehman A, McConnell P, Galantowicz M, Higgins R, Whitson B. Contemporary Analysis of the Effect of Induction Immunosuppression of Survival in Pediatric Lung Transplant Recipients, A [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/contemporary-analysis-of-the-effect-of-induction-immunosuppression-of-survival-in-pediatric-lung-transplant-recipients-a/. Accessed March 6, 2026.« Back to 2013 American Transplant Congress
