Comparison of Voclosporin and Tacrolimus 1 Year Outcomes as Predicted by PKPD in the PROMISE Trial, A
University of Cincinatti, Cincinnati, OH
Penn Medicine, Philadelphia, PA
University of Alabama Birmingham, Birmingham, AL
Newark Beth Israel Medical Center, West Orange, NJ
Henry Ford Hospital, Detriot, MI
Isotechnika Pharma, Edmonton, AB, Canada
Stanford, Palo Alto, CA
Meeting: 2013 American Transplant Congress
Abstract number: B1018
Objective:
To determine the PKPD of VCS, a new calcineurin inhibitor (CNi), compared to TAC in renal allograft patients and to explore PKPD parameters as predictors of clinical outcomes.
Methods:
PKPD analysis was performed in renal allograft patients (receiving anti-CD25 antibody induction, CNi, MMF, prednisone) by linking whole blood drug concentrations to calcineurin activity (CNa). Drug concentrations and CNa were determined using validated LS/MS and P32-radiolabeled assay, and assessed as predictors of clinical outcome by ANOVA. Clinical outcomes (1 year post-transplant) were biopsy proven acute rejection (BPAR), new-onset diabetes after transplantation (NODAT), moderate/severe infection as assessed by the investigator, and OPTIMAL (lack of BPAR, NODAT, or INFECTION).
Results:
VCS trough concentrations demonstrated statistically significant differences for OPTIMAL, NODAT and BPAR. TAC trough concentrations for all groups were nearly identical, suggesting little separation between BPAR and NODAT concentrations.
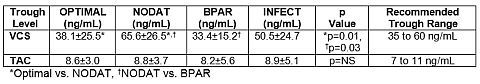
CNa in the VCS group demonstrated a statistically significant difference between OPTIMAL and BPAR (1. 1 ± 0.5 pmol/min/mg vs. 1.5 ± 0.6 pmol/min/mg (p = 0.01)). CNa in the TAC group only approached statistical significance when the BPAR and NODAT groups were compared (1.6 ± 0.5 pmol/min/mg vs. 0.9 ±0.4 pmol/min/mg (p = 0.06)). TAC CNa for OPTIMAL compared to BPAR was 1.11 ± 0.37 pmol/min/mg vs. 1.6 ± 0.5 pmol/min/mg.
Conclusions:
This analysis of patients enrolled in the PROMISE study demonstrated a clear separation between efficacy (BPAR) and toxicity (NODAT, INFECTION). This separation between efficacy and toxicity was not seen with TAC exposure. It may thus be possible to optimize patients’ outcomes prospectively by targeting a VCS exposure of trough concentrations between 35 to 60 ng/mL.
Mayo, P.: Employee, Isotechnika.
To cite this abstract in AMA style:
Alloway R, Bloom R, Gaston R, Goggins M, Mayo P, Busque S. Comparison of Voclosporin and Tacrolimus 1 Year Outcomes as Predicted by PKPD in the PROMISE Trial, A [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/comparison-of-voclosporin-and-tacrolimus-1-year-outcomes-as-predicted-by-pkpd-in-the-promise-trial-a/. Accessed February 27, 2026.« Back to 2013 American Transplant Congress
