Comparison of Statin-Associated Adverse Events Across Different Types of Statins in Kidney Transplant Recipients
S. Bae1, J. Ahn2, M. A. Schnitzler3, K. Lentine4, D. Segev1, M. McAdams-DeMarco1
1Johns Hopkins University, Baltimore, MD, 2SOM, Johns Hopkins University, Baltimore, MD, 3Surgery, Saint Louis University, St. Louis, MO, 4Saint Louis University, St. Louis, MO
Meeting: 2022 American Transplant Congress
Abstract number: 784
Keywords: Safety
Topic: Clinical Science » Kidney » 35 - Kidney: Cardiovascular and Metabolic Complications
Session Information
Session Name: Kidney: Cardiovascular and Metabolic Complications
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
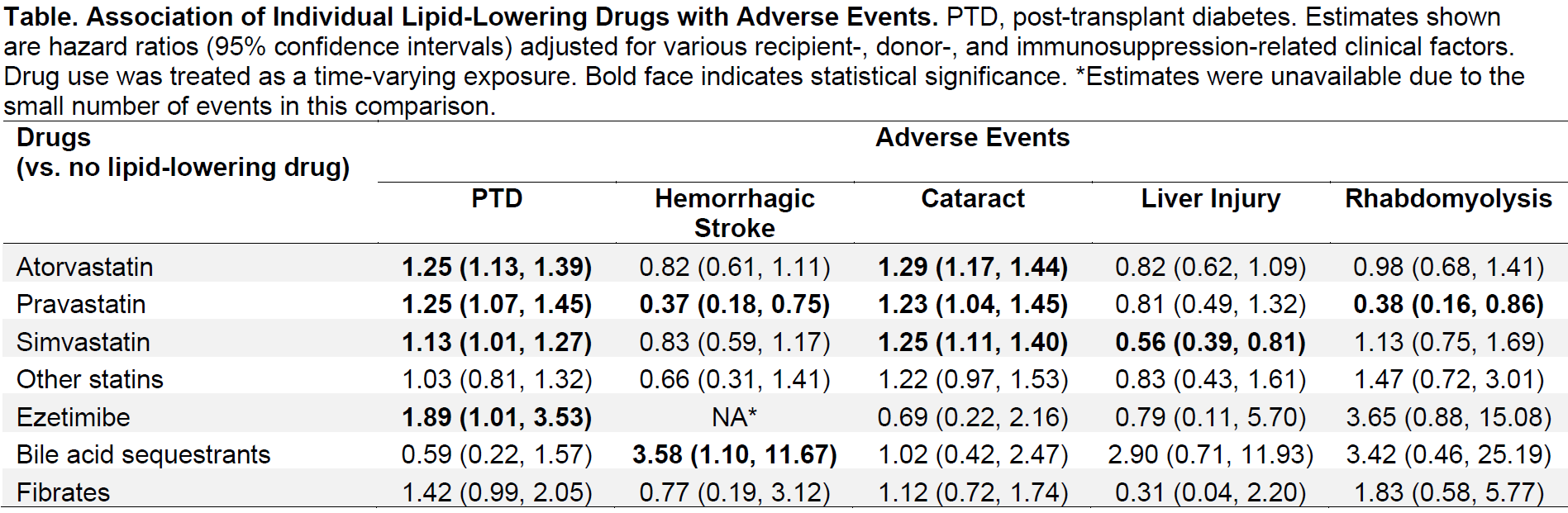
*Purpose: Statins are the third most prescribed drug class in kidney transplant (KT) recipients. Statin’s safety profile in KT recipients remains unclear, although these individuals are uniquely burdened by concomitant immunosuppression and cardio-renal comorbidities. We conducted a national study to characterize the association of statin use with potential adverse events in KT recipients.
*Methods: We studied adult (≥18) single-organ KT recipients in 2006-2016 who used Medicare as primary payer (n=82,998). We used prescription drug claims to capture statin use, and ICD-9/10 diagnosis codes to capture statin-related adverse events, including new-onset diabetes after transplantation (NODAT), hemorrhagic stroke, cataract, liver injury, and rhabdomyolysis. We separately categorized non-statin lipid-lowering drugs (ezetimibe, bile acid sequestrants, and fibrates) as active comparators. We conducted Cox regression for each outcome with statin use as a time-varying exposure.
*Results: Statin use steadily increased over the study period (35.4% at KT, 51.0% at 3-years post-KT, and 54.1% at 5-years post-KT). Overall, NODAT was the most common adverse event (5-year incidence=33.6%), followed by cataract (15.4%), liver injury (2.2%), hemorrhagic stroke (1.6%), and rhabdomyolysis (1.2%). Compared to non-users (Table), statin users had higher hazard of NODAT (aHR=1.28 [95% CI, 1.22-1.34]) and cataract (aHR=1.27 [1.21-1.33]), but lower hazard of hemorrhagic stroke (aHR=0.66 [0.57-0.76]) and liver injury (aHR=0.79 [0.69-0.90]). These associations varied slightly across statin types (e.g., NODAT, atorvastatin, aHR=1.25 [1.13-1.39]; simvastatin, aHR=1.13 [1.01-1.27]; and other statins, aHR=1.03 [0.81-1.32]; vs. no lipid-lowering drug).
*Conclusions: While statins appear to be generally safe in KT recipients, the 1.28-fold hazard of NODAT associated with statin use is non-trivial considering the importance of NODAT prevention in this population. A careful risk-benefit assessment might be warranted in recipients with high diabetes risk.
To cite this abstract in AMA style:
Bae S, Ahn J, Schnitzler MA, Lentine K, Segev D, McAdams-DeMarco M. Comparison of Statin-Associated Adverse Events Across Different Types of Statins in Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/comparison-of-statin-associated-adverse-events-across-different-types-of-statins-in-kidney-transplant-recipients/. Accessed February 20, 2026.« Back to 2022 American Transplant Congress