Comparing Bortezomib Protocol versus IVIG/Rituximab in the Treatment of Antibody Mediated Rejection (AMR) in Kidney Transplantation
1Pharmacy, Jackson Memorial Hospital/Miami Transplant Institute, Miami, FL, 2Nephrology & Hypertension, Miami Transplant Institute, Jackson Memorial Hospital/Miami Transplant Institute, Miami, FL
Meeting: 2021 American Transplant Congress
Abstract number: 1036
Keywords: Antibodies, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » Kidney Acute Antibody Mediated Rejection
Session Information
Session Name: Kidney Acute Antibody Mediated Rejection
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: To compare outcomes of historical treatment of AMR [PLEX + IVIG + Rituximab] with addition of bortezomib, along with characterizing safety parameters aimed at evaluating the risk versus benefit of additional immunosuppression.
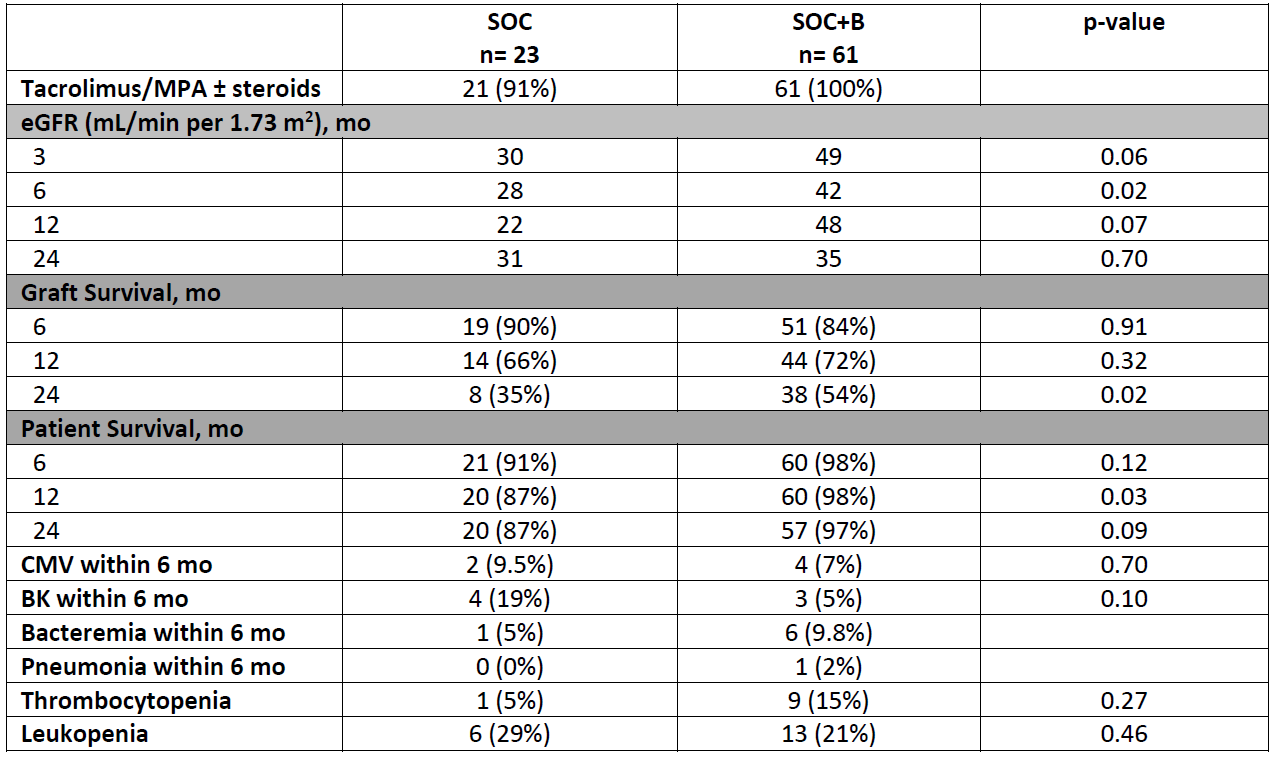
*Methods: A single-center retrospective chart review was performed on patients receiving treatment for AMR with either Standard of Care (SOC) [Plasmaphereis (PLEX) x 3-7 sessions + Intravenous Immunoglobulin (IVIG) 2g/kg + Rituximab (RTX) 375 mg/m2 x 1] or SOC + Bortezomib 1.3 mg/m2 x 4 doses with IVIG (0.1 g/kg) after PLEX (SOC+B) from 11/2012 to 11/2017. Adult renal transplant recipients (RTR) were included upon first biopsy proven acute AMR. Combined organ transplants, recurrent AMR, AMR > 3 years post-transplant, and those transplanted outside our institution were excluded. Primary outcomes included eGFR over 3, 6, 12, and 24 months, graft and patient survival at 6, 12, and 24 months post treatment. Secondary outcomes included incidence of bacteremia and/or pneumonia within 6 months of treatment, BK or CMV viremia within 6 months, and dose-limiting side effects including thrombocytopenia and leukopenia.
*Results: Total of 84 patients were included, SOC (n=23) and SOC+B (n=61). AMR occurred a median of 1.19 years post-transplant. Majority of patients in both groups received maintenance with Tacrolimus/Mycophenolate (MPA) ± steroids. SOC+B group had a trend towards higher median eGFR at time of biopsy (21 vs 36 mL/min per 1.73 m2; p=0.051), with eGFR at 6 months significantly higher (28 vs 42 mL/min per 1.73 m2, p=.02). Graft survival at 24 months was higher in SOC+B (35% versus 54%; p=0.02) as well as patient survival at 12 months (87% vs 98%; p=0.03). No difference was observed in incidence of bacteremia, BK or CMV between groups.
*Conclusions: We observed higher eGFR at 6 months, patient survival at 12 months and graft survival at 24 months with the addition of bortezomib to our SOC in AMR. No significant difference was seen in infectious or safety profile between groups. Further detailed multivariable analysis is needed to confirm these findings.
To cite this abstract in AMA style:
Lowery VM, Centeno A, Harrison T, Cabrera P, Mattiazzi A. Comparing Bortezomib Protocol versus IVIG/Rituximab in the Treatment of Antibody Mediated Rejection (AMR) in Kidney Transplantation [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/comparing-bortezomib-protocol-versus-ivig-rituximab-in-the-treatment-of-antibody-mediated-rejection-amr-in-kidney-transplantation/. Accessed February 20, 2026.« Back to 2021 American Transplant Congress