CNI-Related Renal Impairment in Liver Transplantation: A Comparison of Cyclosporine and Tacrolimus-Based Regimens
OptumInsight, Uxbridge, United Kingdom
OptumInsight, Nanterre, France
Wellmera AG, Basel, Switzerland
Novartis Pharma AG, Basel, Switzerland
Novartis Healthcare Pvt. Ltd., Hyderabad, India
University of Minnesota, MN
Meeting: 2013 American Transplant Congress
Abstract number: D1700
Background: Long-term and/or high dose exposure to CNI (cyclosporine [CsA] and tacrolimus [Tac]) is associated with high incidence of nephrotoxicity and chronic kidney disease (CKD).
Objective: To compare the renal safety of CNI-based immunosuppression (IS) regimens post-LTx.
Methods: We conducted a systematic review of the published literature for randomised controlled trials and observational studies reporting efficacy and safety outcomes of IS regimens post-LTx. A fixed-effect mixed-treatment network analysis was used to compute pooled estimates of the change in estimated glomerular filtration rate (eGFR) at 12 months from baseline (day of LTx).
Results: A total of 1,043 publications were identified, 77 met the pre-defined eligibility criteria, of which 11 evaluated the effect of CsA relative to Tac on renal function (RF). Seven publications estimated the impact on renal function (Figure 1).
In most studies no differences were observed between CNI regimens with regard to RF. A mixed treatment comparison confirmed no significant difference in mean change from baseline between CsA and Tac monotherapies (-15.55; 95% credible interval [Crl]: -41.90 – +10.90) and with azathioprine (-6.37; 95% Crl: -18.00 – +5.43). In studies comparing Tac to CsA microemulsion (CsA-ME; Neoral) the results are inconclusive as Charco (2002) & Niel (2009) reported better RF with Tac, whereas Van Buren (1998), O’Grady (2002; data not shown) and Levy (2006; data not shown) found no differences between the two CNI-regimens.
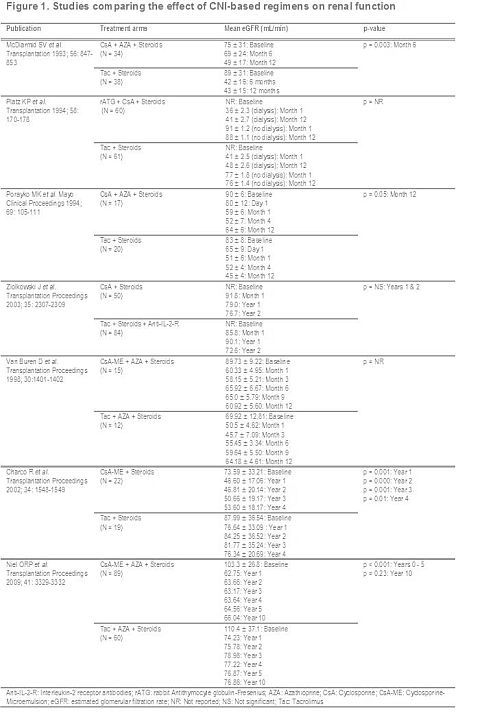
Conclusion: Overall, the published evidence shows CsA- and Tac-based regimens are similar with respect to their impact on RF in liver transplant recipients. Further research is required to establish the optimal use of CNIs with regard to lessening nephrotoxicity.
Davie, A.: Other, Neoral (Cyclosporine), Contracted as an External Consultant. Damera, V.: Other, Neoral (Cyclosporine), Contracted as an External Consultant. Bianic, F.: Other, Neoral (Cyclosporine), Contracted as an External Consultant. Ricci, J.: Other, Neoral (Cyclosporine), Contracted as an External Consultant. Bernhardt, P.: Employee, Neoral (Cyclosporine). Gokhale, S.: Employee, Neoral (Cyclosporine). Lake, J.: Other, Neoral (Cyclosporine), Contracted as a Clinical Advisor.
To cite this abstract in AMA style:
Davie A, Damera V, Bianic F, Ricci J, Bernhardt P, Gokhale S, Lake J. CNI-Related Renal Impairment in Liver Transplantation: A Comparison of Cyclosporine and Tacrolimus-Based Regimens [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/cni-related-renal-impairment-in-liver-transplantation-a-comparison-of-cyclosporine-and-tacrolimus-based-regimens/. Accessed March 9, 2026.« Back to 2013 American Transplant Congress
