Clinical Outcomes of Kidney Transplant Recipients Infected with Hepatitis C Virus and Treated with Direct-Acting Antiviral Therapy
M. Alkadi,1 E. Abuhelaiqa,1 M. Derbala,2 O. Fituri,1 J. Mahmoud,3 M. Jarman,3 M. Asim,1 A. Aziz,1 A. Nauman,1 A. Hamdi,1 Y. Medhat,2 S. Al Kaabi,2 H. Al-Malki.1
1Medicine-Nephrology, Hamad Medical Corporation, Doha, Qatar
2Medicine-Gastroenterology, Hamad Medical Corporation, Doha, Qatar
3Surgery-Transplant, Hamad Medical Corporation, Doha, Qatar.
Meeting: 2018 American Transplant Congress
Abstract number: A188
Keywords: Hepatitis C, Infection, Kidney transplantation, Outcome
Session Information
Session Name: Poster Session A: Kidney Transplant Goes Viral
Session Type: Poster Session
Date: Saturday, June 2, 2018
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall 4EF
Introduction:
Hepatitis C virus (HCV) infection has negative impact on patient and graft survival in kidney transplant recipients. In the past, treatment of HCV infection post transplantation was challenging given low virologic response rate and high risk of acute rejection. New direct-acting antiviral therapy (DAA) has revolutionized treatment of HCV infection. The aim of this retrospective study was to determine the clinical outcomes of kidney transplant recipients treated with DAA therapy at our center.
Methods:
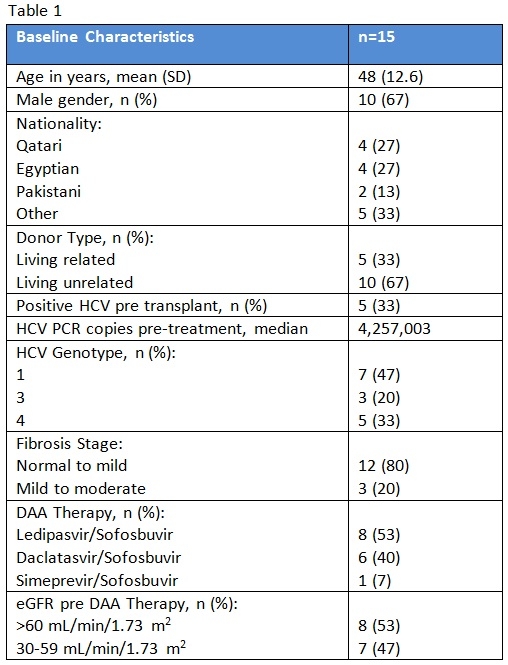
Out of 753 transplant recipients followed at our center, 15 patients were found to have positive HCV PCR post transplantation and were treated with DDA therapy.
Results:
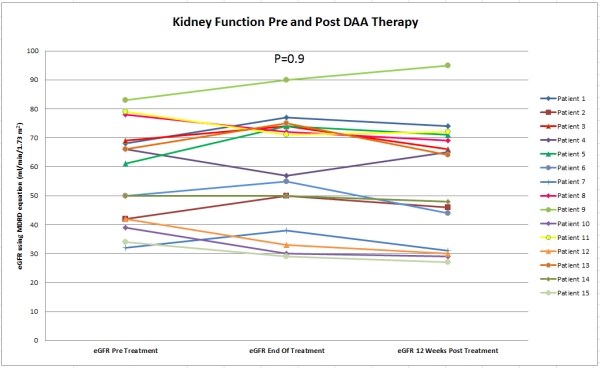
All patients were treated with sofosbuvir-based regimen. Only 1 patient was treated with ribavirin in addition to DAA therapy. Two other patients were co-infected with hepatitis B virus and were treated with entecavir as well. The duration of DAA treatment was ranging between 3 to 6 months. None of the treated patients had HIV infection. Baseline characteristics of patients are summarized in table 1. All patients had undetected HCV PCR by the end of treatment and had 12-week sustained virologic response. There were no major side effects associated with DAA therapy and kidney function remained stable post treatment as shown in figure 1.
Figure 1
Conclusion:
100% of our kidney-transplant recipients achieved 12 week sustained virologic response post DAA therapy. We found treatment with DAA therapy effective and safe with no increased risk of graft dysfunction.
CITATION INFORMATION: Alkadi M., Abuhelaiqa E., Derbala M., Fituri O., Mahmoud J., Jarman M., Asim M., Aziz A., Nauman A., Hamdi A., Medhat Y., Al Kaabi S., Al-Malki H. Clinical Outcomes of Kidney Transplant Recipients Infected with Hepatitis C Virus and Treated with Direct-Acting Antiviral Therapy Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Alkadi M, Abuhelaiqa E, Derbala M, Fituri O, Mahmoud J, Jarman M, Asim M, Aziz A, Nauman A, Hamdi A, Medhat Y, Kaabi SAl, Al-Malki H. Clinical Outcomes of Kidney Transplant Recipients Infected with Hepatitis C Virus and Treated with Direct-Acting Antiviral Therapy [abstract]. https://atcmeetingabstracts.com/abstract/clinical-outcomes-of-kidney-transplant-recipients-infected-with-hepatitis-c-virus-and-treated-with-direct-acting-antiviral-therapy/. Accessed February 26, 2026.« Back to 2018 American Transplant Congress