Clinical Outcomes In A Large Transplant Cohort Hospitalized With COVID-19: Mycophenolate Mofetil (MMF) Utilization And Mortality Trends
1Baylor Scott & White Research Institute, Dallas, TX, 2Baylor University Medical Center, Dallas, TX, 3Gilead Sciences, Foster City, CA, 4Certara, New York, NY
Meeting: 2022 American Transplant Congress
Abstract number: 9062
Keywords: COVID-19, Immunosuppression, Kidney/liver transplantation, Mycophenolate mofetil
Topic: Basic & Clinical Science » Basic & Clinical Science » 73 - COVID-19
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
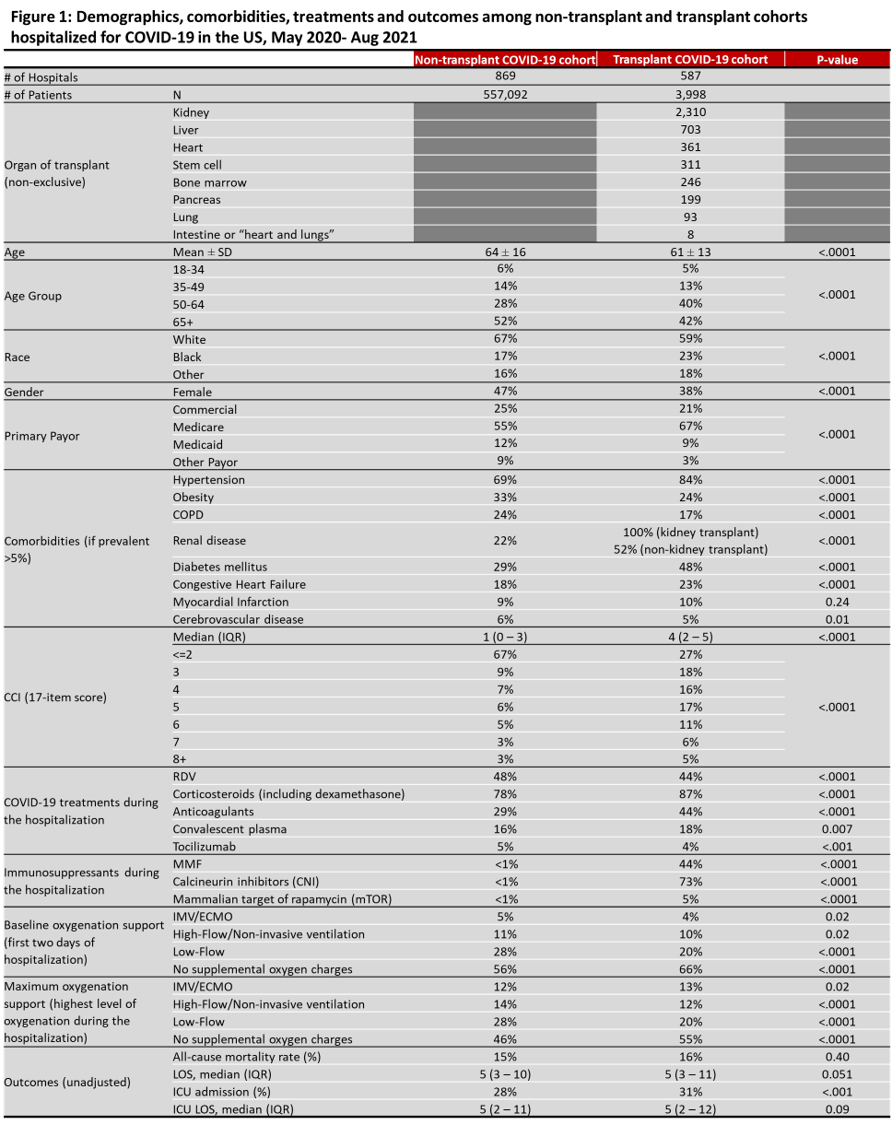
*Purpose: To characterize demographics, treatment patterns, and outcomes among 3,998 transplant patients hospitalized for COVID-19 over 16 months of the pandemic (May ’20-Aug ’21).
*Methods: Adult patients in a transplant cohort (TC) and non-transplant cohort (NTC) hospitalized with COVID-19 (ICD-10: U07.1) were compared in the Premier Healthcare Database from May ’20-Aug ’21. Baseline measures in first two days, demographics, comorbidity, COVID-19 treatments and immunosuppressants were analyzed. Outcomes included mortality (discharge status expired or hospice) and hospital and ICU LOS.
*Results: 3,998 TC patients were hospitalized for COVID-19 in 587 US hospitals. Compared to NTC, TC were younger (61 vs 64 yrs; p<.0001), less likely to be white (59% vs 67%; p<.0001), obese (24% vs 33%; p<.0001) or have COPD (17% vs 24%; p<.0001). TC had higher rates hypertension (84% vs 69%; p<.0001), renal disease (80% vs 22%, p<.0001), diabetes (48% vs 29%; p<.0001) and chronic heart failure (23% vs 18%; p<.0001). During hospitalization, a lower proportion of TC needed any oxygen therapy compared to NTC (p<.05). Compared to NTC, fewer TC received remdesivir (RDV) (44% vs 48%; p<.0001), but more received corticosteroids (87% vs 78%; p<.0001), anticoagulants (44% vs 29%; p<.0001) and convalescent plasma (18% vs 16%; p=0.007). In TC, 44% received MMF, 73% calcineurin inhibitors and 5% mTOR. Use of MMF did not change over time (43% May-Jul 2020; 43% Aug-Dec 2020; 45% 2021). TC had higher ICU admission rates (31% vs 28%; p.001), but similar hospital LOS and ICU LOS compared to NTC. All-cause mortality in NTC (15% overall; 16% May-Jul 2020; 16% Aug-Dec 2020; 14% 2021) was not significantly different than TC over time (16% overall; 13% May-Jul 2020; 16% Aug-Dec 2020; 16% 2021).
*Conclusions: Very few large studies have assessed COVID-19 management in transplant patients over time. All-cause mortality was comparable in both cohorts despite TC immunosuppression. RDV use was lower in TC. Uncertainty around MMF use in COVID-19 patients did not impact reported use of MMF. Further analyses are needed to evaluate confounding factors (medication sequence, time since transplant, disease severity) and impact of external factors such as earlier testing and treatment for COVID-19, vaccination, and new variants.
To cite this abstract in AMA style:
Gottlieb RL, Askar M, Chen L, Chandak A, Mozaffari E, Thrun M, Haubrich R. Clinical Outcomes In A Large Transplant Cohort Hospitalized With COVID-19: Mycophenolate Mofetil (MMF) Utilization And Mortality Trends [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcomes-in-a-large-transplant-cohort-hospitalized-with-covid-19-mycophenolate-mofetil-mmf-utilization-and-mortality-trends/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress