Belatacept Removal by Plasmapheresis: Kinetics, Dose Adjustments and Clinical Recommendations
1U Cincinnati, Cincinnati, OH, 2Cincinnati Children's, Cincinnati, OH, 3U Cincinnati / Christ Hospital, Cincinnati, OH, 4Christ Hospital, Cincinnati, OH
Meeting: 2020 American Transplant Congress
Abstract number: 606
Keywords: Co-stimulation, Immunosuppression, Pharmacokinetics, Plasmapheresis
Session Information
Session Name: All Organs: Pharmacogenomics / Pharmacokinetics
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:51pm-4:03pm
Presentation Time: 3:51pm-4:03pm
Location: Virtual
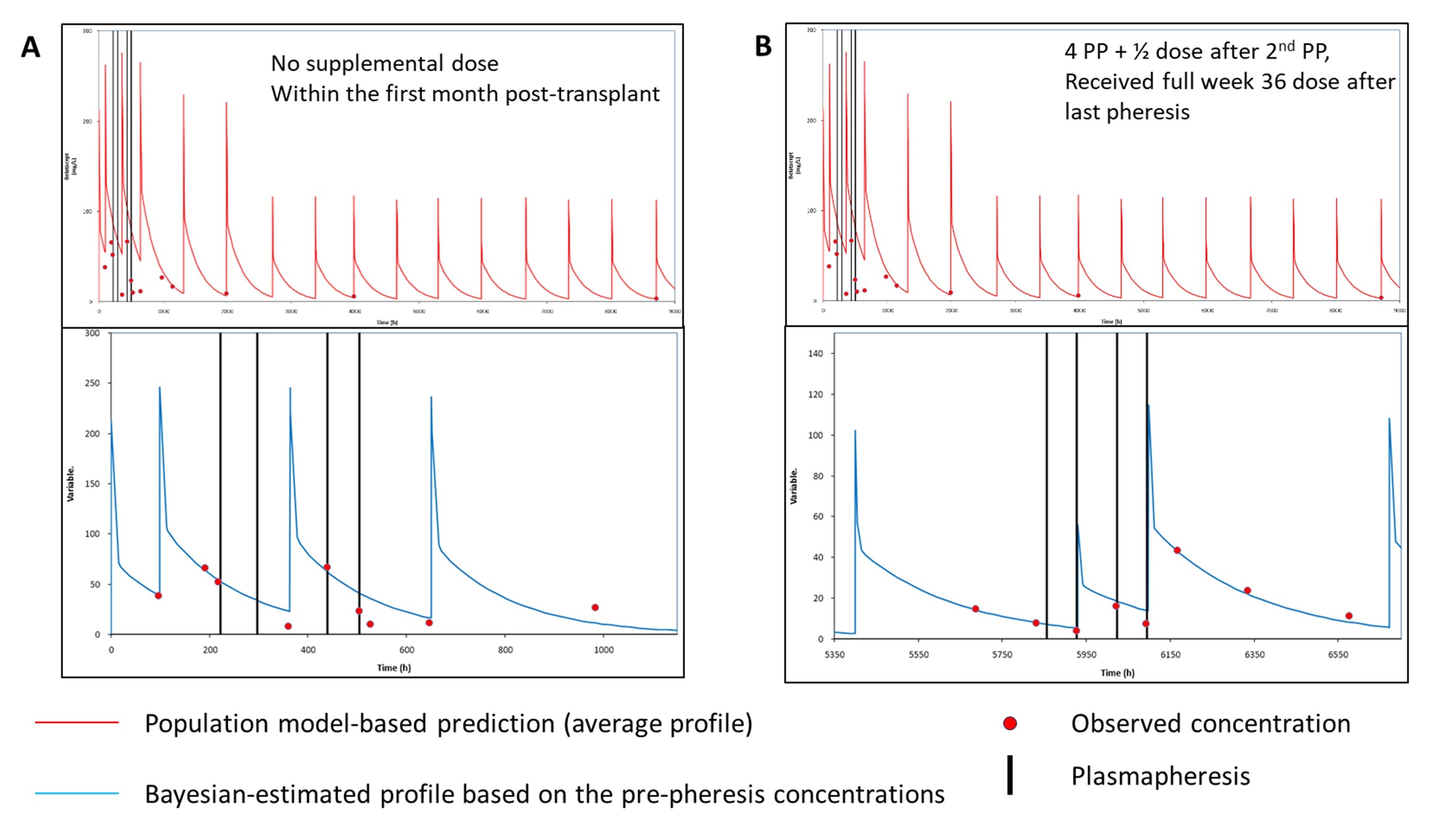
*Purpose: Belatacept (BELA), a protein immunosuppressant, has limited available pharmacokinetic (PK) data. Available PK data, limited to product development analyses, shows moderate intrapatient variability with a defined therapeutic upper limit serum concentration, which support dosing on a fixed mg/kg basis with no therapeutic drug monitoring (TDM) strategy. However, clinical scenarios arise when BELA TDM may be beneficial, ie rejection, infectious toxicity, plasmapheresis (PP). This report evaluates the impact of PP and supplemental post PP BELA dosing on BELA serum levels.
*Methods: Kidney transplant patients receiving denovo BELA per package insert and receiving PP for clinical cause were retrospectively evaluated. Each PP cycle consisted of a 1.5 x plasma volume exchange over ~2.5 hours. During the first post transplant month, a 50% supplemental BELA dose was administered after every 2 PP cycles with a full dose upon PP completion. Patients one month post transplant, a 50% supplemental BELA dose was administered after every 2 PP cycles with a full dose resumed based upon time of next BELA dose. Available serum samples were analyzed for BELA concentrations by ELISA. All dosing data, body weight and BELA concentration measurements were entered into the MW/Pharm++ (Mediware). Individual PK profiles were by Bayesian estimation using concentrations collected immediately before PP using a population PK model. Plasma concentrations collected after PP events were compared with the model-based predicted profile based on the pre-PP concentrations.
*Results: Seven kidney transplant underwent PP (range 1-4 sessions), 3 within the 1st month from transplant and 3 did not receive supplemental BELA doses after PP. Patients undergoing PP within the 1st month had lower-than-predicted (population and Bayesian, Figure 1A) BELA concentrations, especially if they did not receive supplemental dosing. This impact was not as pronounced for patients who were PP more than one month from transplant and who received supplemental dosing (Figure 1B).
*Conclusions: PP seems to have a significant impact on BELA concentrations, which may be more pronounced during the first month of therapy and when no supplemental doses are administered. Administering ½ dose of BELA after every 2nd PP session while maintaining patients on their regular dosing schedule may mitigate impact of PP, particularly after the initial 10mg/kg dosing phase.
To cite this abstract in AMA style:
Tremblay S, Wilson N, Miyagawa B, Kuzaro H, Shields AR, Alloway RR, Govil A, Kremer J, Woodle E, Mizuno T, Vinks AA. Belatacept Removal by Plasmapheresis: Kinetics, Dose Adjustments and Clinical Recommendations [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-removal-by-plasmapheresis-kinetics-dose-adjustments-and-clinical-recommendations/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress