Belatacept Exposure Not Associated With Rejection. Can Doses be Lowered without Compromising Efficacy?
1U Cincinnati, Cincinnati, OH, 2Cincinnati Children's Med Center, Cincinnati, OH, 3U Illinois, Chicago, IL, 4Tampa Gen, Tampa, FL, 5U Wisconsin, Madison, OH, 6Centura Transplant, Denver, CO, 7U Minnesota, Minneapolis, MN
Meeting: 2021 American Transplant Congress
Abstract number: 233
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation, Pharmacokinetics
Topic: Clinical Science » Kidney » Kidney: Acute Cellular Rejection
Session Information
Session Name: Kidney: Acute Cellular Rejection
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:30pm-4:35pm
Presentation Time: 4:30pm-4:35pm
Location: Virtual
*Purpose: Phase 1 and 2 belatacept(BELA) pharmacokinetic(PK) studies informed dosing in phase 3 studies. PK data from these studies revealed higher BELA troughs on Day 5 were associated with lower acute rejection (AR) at 1mo, but no other relationship between PK and AR was observed. AR results were similar in BELA-based Early Steroid-withdrawal Trial (BEST). BEST samples explored associations between BELA PK and AR under depleting induction.
*Methods: Prospectively collected samples were used to retrospectively analyze BELA troughs via a validated quantitative enzyme-linked immunoassay. BELA clearance (Cl) was estimated using Bayesian estimation with published population PK model as the Bayesian prior(Zhou et al) with the clinical software MWPharm+(Mediware). Allometric scaling accounted for weight differences. Individual patient profiles estimated troughs, cumulative area under the curve(AUC) and average concentration(Cavg) during BELA dosing. PK parameters and Cavg at time of AR were analyzed for AR and recurrent AR.
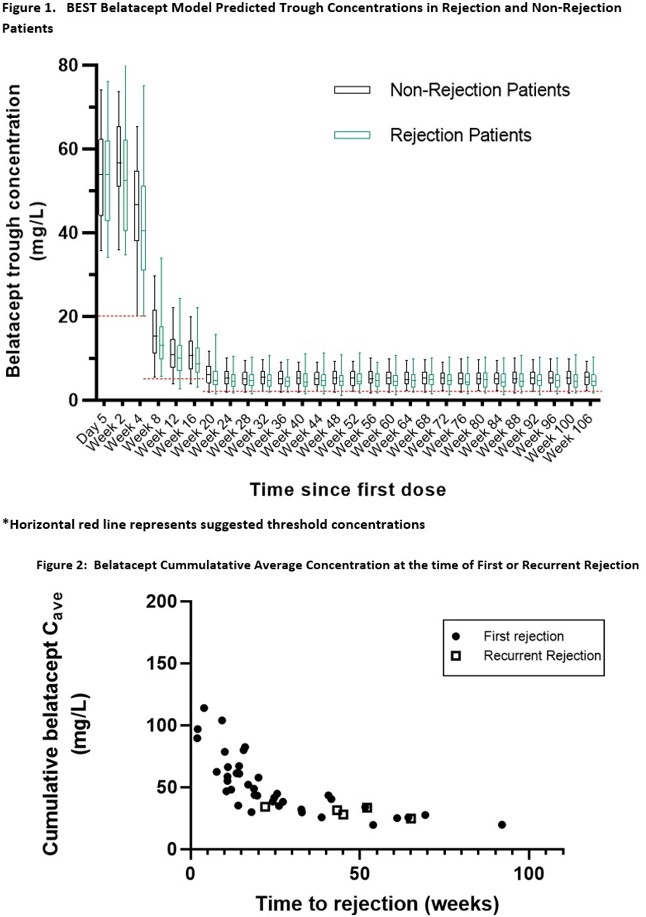
*Results: 876 BELA troughs in 191 patients(pts) were modeled. Results showed agreement between observed troughs and both population model-predicted and Bayesian estimated concentrations (R2=0.84 and 0.88). BELA exposure was no different in rejecting or non rejecting pts(Fig1). No differences in BELA PK parameters were observed based on induction. Inter-individual variability in Cl was low (CV=22%), and closely matched reported Cl. 46(24%) pts had AR and 10(5.2%) pts had recurrent AR. Mean time to AR was 174 (±149) days with 63% of AR prior to 6mo and 26% between 6-12mo. There were no significant differences between allometrically standardized Cl in pts with AR or recurrent AR compared to those without. Cavg and AUC with AR were not significantly different at any time points except for significantly lower Cavg and AUC at 6-12mo in AR. The cumulative Cavg(Fig2) or AUC at time of AR event was influenced by time post transplant.
*Conclusions: At labeled BELA doses, Cl, Cavg, and AUC do not appear to correlate with AR. The Cavg observed were significantly higher than threshold and observed concentrations previously reported, especially during the induction phase. Data suggests that higher BELA exposure does not improve AR rates and alternative dosing strategies may be explored.
To cite this abstract in AMA style:
McGowan M, Bickenbach A, Miyagawa B, Christianson A, Mizuno T, West-Thielke P, Leone J, Woodle E, Kaufman D, Wiseman A, Matas A, Vinks A, Alloway R. Belatacept Exposure Not Associated With Rejection. Can Doses be Lowered without Compromising Efficacy? [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-exposure-not-associated-with-rejection-can-doses-be-lowered-without-compromising-efficacy/. Accessed February 19, 2026.« Back to 2021 American Transplant Congress