Belatacept Every Two Month Maintenance Dosing in Kidney Transplantation: A Randomized, Non-Inferiority Trial
Emory University School of Medicine, Atlanta, GA
Meeting: 2020 American Transplant Congress
Abstract number: 332
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation
Session Information
Session Name: Immunosuppressive Drug Minimization
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: Maintenance immunosuppression with belatacept following kidney transplantation results in a reduced risk of patient death and graft loss as compared to calcineurin inhibitors. However, broad application of belatacept has been in part limited by higher early rejection rates, protective immunity concerns, and logistical barriers related to a monthly (q1m) IV infusion requirement. In a phase II clinical study every 2 month (q2m) belatacept was associated with higher acute rejection rates early after transplant but notable stability in renal function over the long-term. Thus q2m dosing of belatacept could reduce access barriers and improve utilization while maintaining renal allograft function.
*Methods: To determine whether q2m dosing of belatacept is noninferior to standard q1m maintenance, we conducted a prospective, single center randomized trial in low immunologic risk, stable renal transplant recipients. First-time recipients on belatacept were randomly assigned to continue on standard maintenance therapy (5 mg/kg) q1m or receive q2m dosing. The primary objective was a noninferiority comparison of renal function at 12 months between the q1m and q2m groups (based on an equivalence margin of 6.0 ml/min/1.73m2).
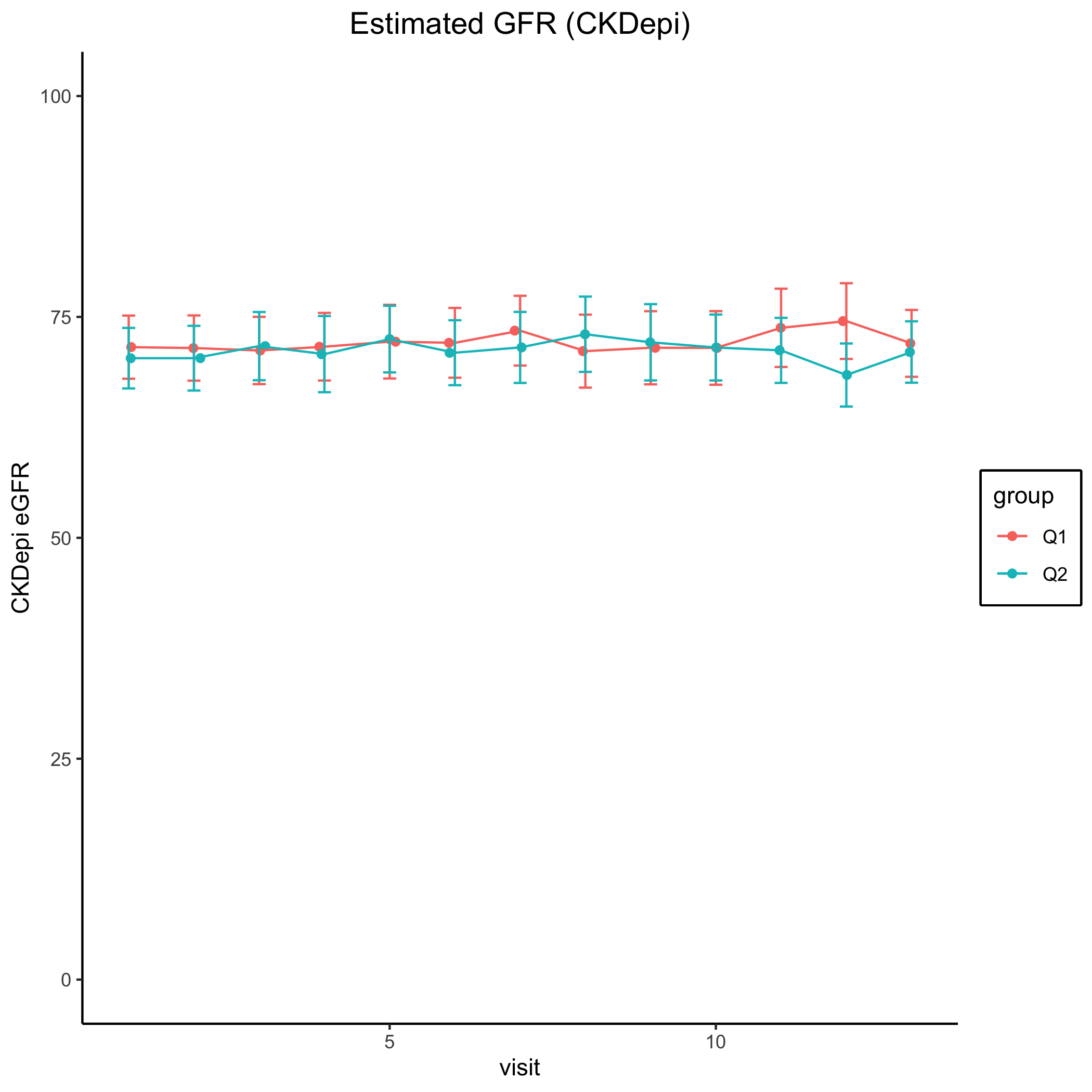
*Results: Of 176 recipients assessed for eligibility, 166 met inclusion criteria and were randomized to q1m control group (n=82) or q2m study group (n=84). Study status is complete and undergoing formal analysis, with 77 q1m and 78 q2m subjects completing the 12 month study period. Continuous mean eGFR over the study period was similar between groups (Figure 1), with mean eGFR at 12 months of 72.0 [±1.91] and 71 [±1.75] ml/min/1.73m2 in the q1m and q2m groups, respectively. One borderline rejection was observed in the q1m group and 4 ACRs in the q2m group. Importantly, 3 of 4 q2m ACRs were related to documented noncompliance. No subjects developed DSA in the q1m group and 4 in the q2m group, 3 of which occurred in the noncompliant patients with ACR. Two patient deaths and graft losses occurred in the q1m group while none were observed in the q2m group.
*Conclusions: Pending formal statistical analysis, observed mean eGFRs for transplant recipients maintained on q2m belatacept maintenance therapy suggest noninferiority to standard q1m dosing. As such, less frequent q2m belatacept may be a viable and safe maintenance immunosuppressive strategy in compliant kidney transplant recipients. (ClinicalTrials.gov NCT02560558)
To cite this abstract in AMA style:
Badell IR, Parsons R, Mead S, Thomas S, Karadkhele G, Pastan S, Larsen C. Belatacept Every Two Month Maintenance Dosing in Kidney Transplantation: A Randomized, Non-Inferiority Trial [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-every-two-month-maintenance-dosing-in-kidney-transplantation-a-randomized-non-inferiority-trial/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress