Belatacept Based Simultaneous Calcineurin Inhibitor Avoidance/Early Corticosteroid Withdrawal is Associated with Low New Onset Diabetes Risk
A. R. Shields1, P. West-Thielke2, E. S. Woodle1, D. B. Kaufman3, J. Lipscomb4, J. P. Leone5, A. Wiseman6, E. King4, A. J. Matas7, R. R. Alloway for the BEST Study Group4
1Transplant Surgery, U of Cincinnati, Cincinnati, OH, 2UIC, Chicago, IL, 3Transplant Surgery, U of Wisconsin, Madison, WI, 4U of Cincinnati, Cincinnati, OH, 5Tampa General, Tampa, FL, 6U of Colorado, Denver, CO, 7U Minnesota, Minneapolis, MN
Meeting: 2019 American Transplant Congress
Abstract number: 118
Keywords: Co-stimulation, Glucocortocoids, Hyperglycemia, Post-transplant diabetes
Session Information
Session Name: Concurrent Session: Kidney: Cardiovascular and Metabolic I
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Ballroom C
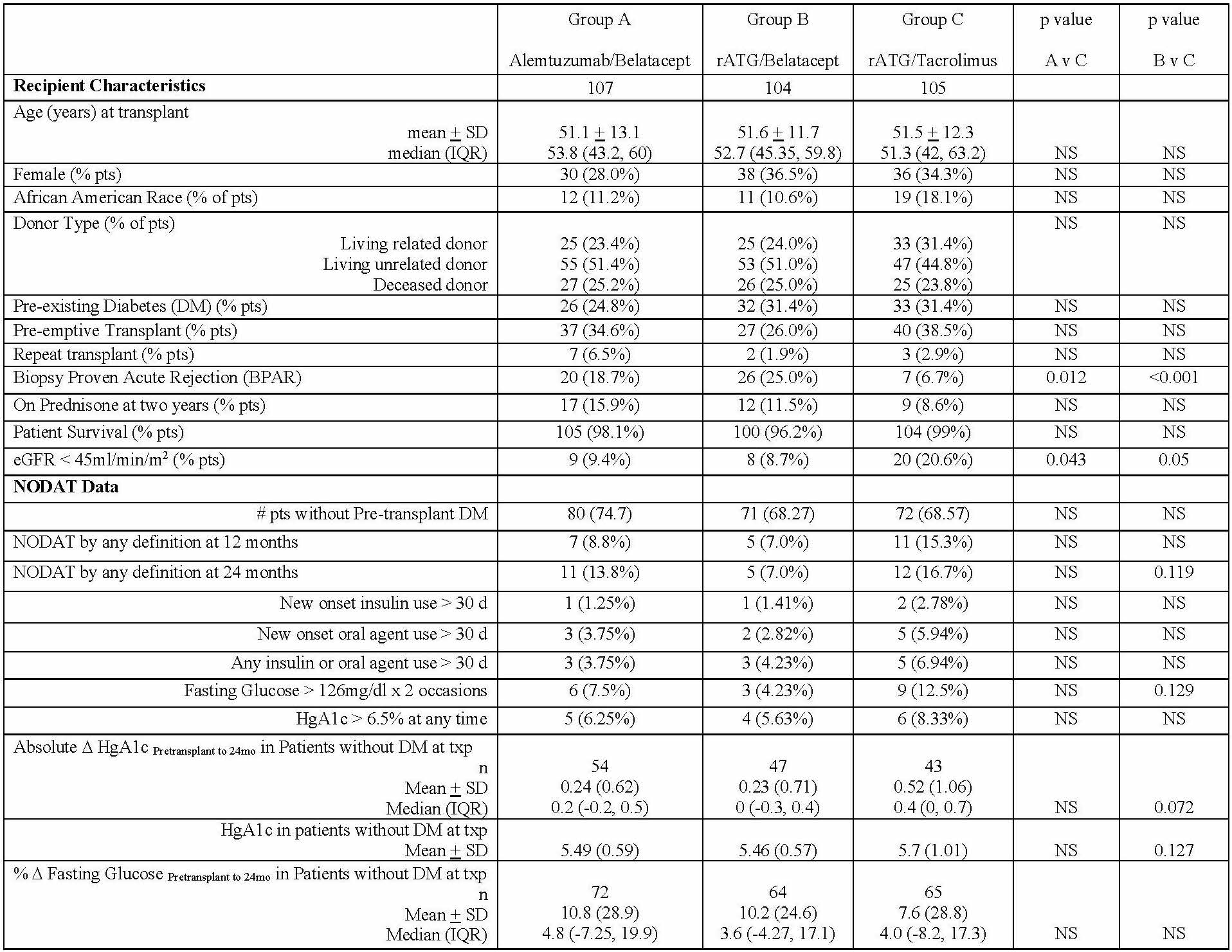
*Purpose: Calcineurin inhibitor (CNI) and/or corticosteroid (CCS) therapy are associated with significant risk of new onset diabetes after transplantation (NODAT). An important consideration for simultaneous CNI avoidance (CNIA) and early corticosteroid withdrawal (ESW) is to reduce NODAT. Recently, simultaneous CNIA/ESW under belatacept was achieved in a large prospective multi-center randomized trial that included rigorous assessment of NODAT using 5 traditional definitions.
*Methods: A prospective, multi-center, randomized, trial was conducted with 3 groups, each of which underwent ESW at five days and received maintenance mycophenolate: Alemtuzumab/belatacept (Group A), rATG/belatacept (Group B), rATG/tacrolimus (Group C). Adult kidney transplants (Ktx) were eligible with the exception of: cPRA>50%, extended criteria donor KTx, EBV seronegative recipients, hepatitis B or C or HIV recipient seropositivity. Data analysis was by intention to treat. NODAT was defined as American Diabetes Association criteria for FBG > 126 mg/dL x 2 consecutive measures or HgA1c ≥ 6.5% or requirement of oral anti-diabetic agents or insulin for ≥ 30 days or any treatment. NODAT was defined at 12 and 24 months post-transplant.
*Results: 316 patients were randomized to the 3 treatment groups with 2-year follow-up and NODAT results are presented in table below. 10.5% of belatacept-based patients developed NODAT (3.9% required therapy) compared to 16.7% of tacrolimus-based patients (6.9% required therapy), p = NS.
*Conclusions: The primary observation from this study is that NODAT incidence by any definition was low in all 3 ESW regimens, with a few parameters that numerically favored belatacept-based simultaneous CNIA/ESW regimens over the tacrolimus-based ESW regimen. It is important to note that this study population was relatively low risk for acute rejection due to inclusion/exclusion criteria. Nevertheless, the results suggest that ESW alone enables achievement of low NODAT rates, and the potential additive contribution of simultaneous CNIA/ESW remains to be demonstrated.
To cite this abstract in AMA style:
Shields AR, West-Thielke P, Woodle ES, Kaufman DB, Lipscomb J, Leone JP, Wiseman A, King E, Matas AJ. Belatacept Based Simultaneous Calcineurin Inhibitor Avoidance/Early Corticosteroid Withdrawal is Associated with Low New Onset Diabetes Risk [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-based-simultaneous-calcineurin-inhibitor-avoidance-early-corticosteroid-withdrawal-is-associated-with-low-new-onset-diabetes-risk/. Accessed March 3, 2026.« Back to 2019 American Transplant Congress