Belatacept-Based, Calcineurin Inhibitor and Corticosteroid Free Immunosuppression: Differential Outcomes Based on Recipient Age
1U Cincinnati, Cincinnati, OH, 2The Christ Hospital, Cincinnati, OH, 3U Wisconsin, Madison, WI, 4U Colorado, Denver, CO, 5Tampa General, Tampa, FL, 6U Minnesota, Minneapolis, MN, 7U Illinois Chicago, Chicago, IL
Meeting: 2020 American Transplant Congress
Abstract number: 114
Keywords: Kidney transplantation
Session Information
Session Name: Novel Immunosuppression: Belatacept
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: Age-based disparities have been described in calcineurin inhibitor (CNI)-based immunosuppression regimens, but are not well-described for belatacept (BELA)-based regimens. This study analyzed the effect of age on outcomes in BELA-based early corticosteroid withdrawal (ESW) regimens from the BEST trial.
*Methods: The BEST trial was a prospective, randomized, multi-center trial that compared two BELA-based, CNI-free, ESW regimens with either alemtuzumab (ALEM) or antithymocyte globulin (rATG) induction to a tacrolimus (TAC)-based ESW regimen with rATG induction. A subgroup analysis was performed for age (<60 vs >60 years). The primary endpoint of the study was patient death, renal allograft loss, or eGFR< 45ml/min/1.73m2.
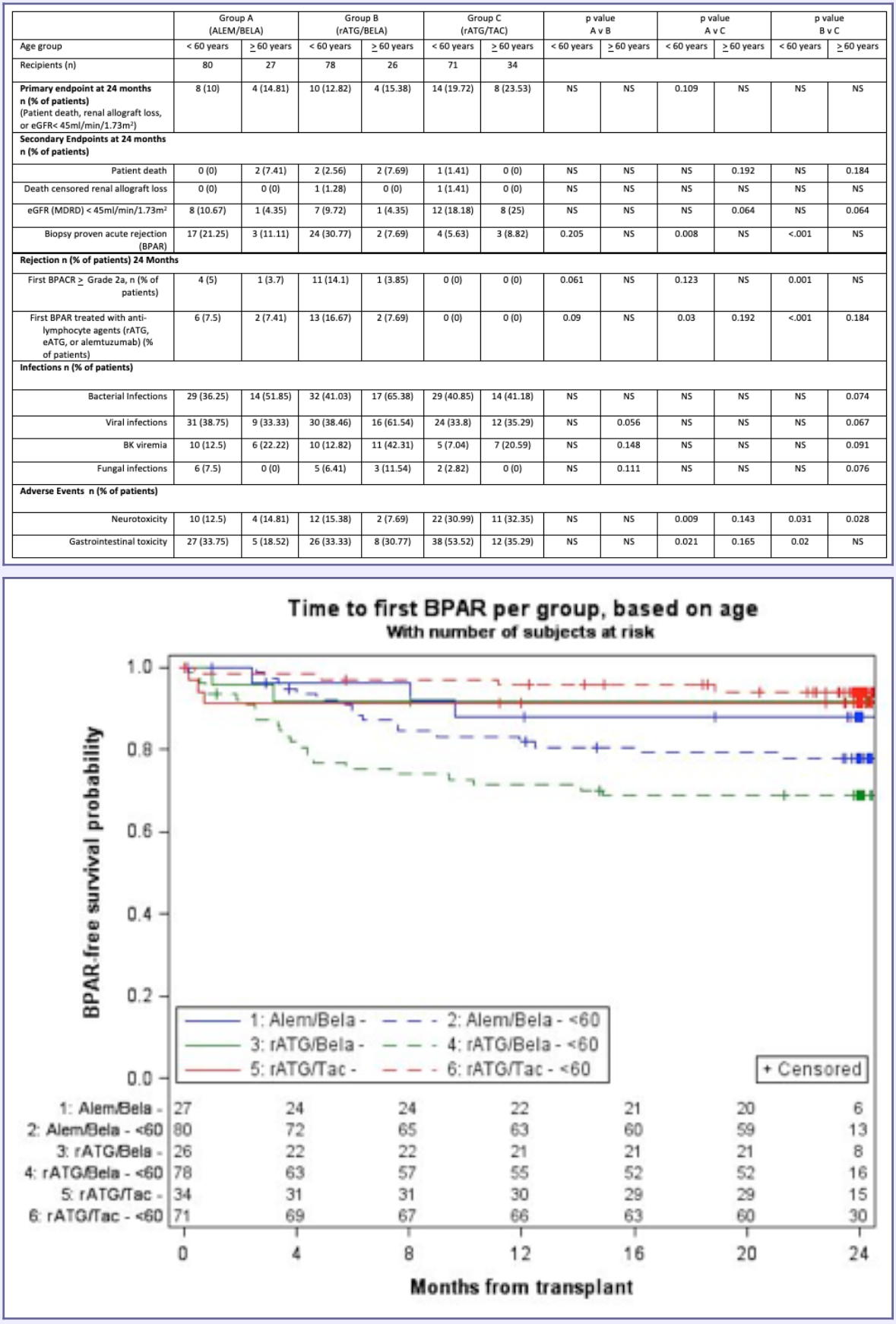
*Results: Eighty-seven patients (27.5% of BEST study patients) were > 60 years old. Demographics between groups were not different. Table 1 summarizes the primary and secondary endpoints of rejection, infection and adverse events by age and treatment group. Time to rejection curves based on treatment group and age are shown in Figure 1. In patients >60 years old, the composite endpoint was not significantly different at 24 months. Notably, significant differences in BPAR were observed in patients < 60 yrs of age; however, the BPAR rate in patients > 60 was not different between BELA groups and the TAC group. Renal function benefit (assessed by eGFR<45 ml/min/1.73 m2) with BELA was also more prominent in patients >60 yrs of age than in those <60 years old. Infections were similar in rATG/TAC and ALEM/BELA groups and trended higher in the rATG/BELA group in older patients. Neurologic toxicities were improved in BELA treatment groups regardless of age.
*Conclusions: Age-based differential responses were observed in BELA/ESW regimens, with patients >60 years old demonstrating similar rejection rates between rATG/TAC and ALEM/BELA and rATG/BELA. Elderly patients appeared to have greater renal function benefits from BELA-based regimens. These data require further exploration in future trials.
To cite this abstract in AMA style:
Wilson N, Shields AR, Kaufman D, Wiseman A, Christianson AL, Tremblay S, Leone JP, Matas AJ, West-Thielke P, Alloway RR, Woodle ES. Belatacept-Based, Calcineurin Inhibitor and Corticosteroid Free Immunosuppression: Differential Outcomes Based on Recipient Age [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-based-calcineurin-inhibitor-and-corticosteroid-free-immunosuppression-differential-outcomes-based-on-recipient-age/. Accessed February 26, 2026.« Back to 2020 American Transplant Congress