Baseline Trends in Tacrolimus Intrapatient Variability in Pediatric and Young Adult Kidney Transplant Recipients
1Pediatric Nephrology, Stanford University, Palo Alto, CA, 2Pediatric Kidney Transplant, Lucile Packard Children's Hospital, Palo Alto, CA, 3Division of Nephrology, Landspitali – the National University Hospital of Iceland, Reykjavik, Iceland
Meeting: 2022 American Transplant Congress
Abstract number: 818
Keywords: Alloantibodies, Kidney transplantation, Pediatric
Topic: Clinical Science » Kidney » 43 - Kidney: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: High tacrolimus intrapatient variability (IPV) is a known risk factor for poor graft outcomes in kidney transplantation. Baseline trends in tacrolimus IPV have not been well-defined in children.
*Methods: Children undergoing kidney-only transplantation from 2010-2018 at Stanford with follow-up time > 1 year were included. Tacrolimus IPV was determined using the coefficient of variation over a 6-month moving window before all tacrolimus levels. De novo donor-specific antibodies (dnDSAs) were identified by routine screening or at investigation of allograft dysfunction. Time-dependent Cox proportional hazards models were used to examine the association between tacrolimus IPV and graft outcomes, where tacrolimus IPV was used as a time-varying categorical variable with IPV < 30% or > 30%.
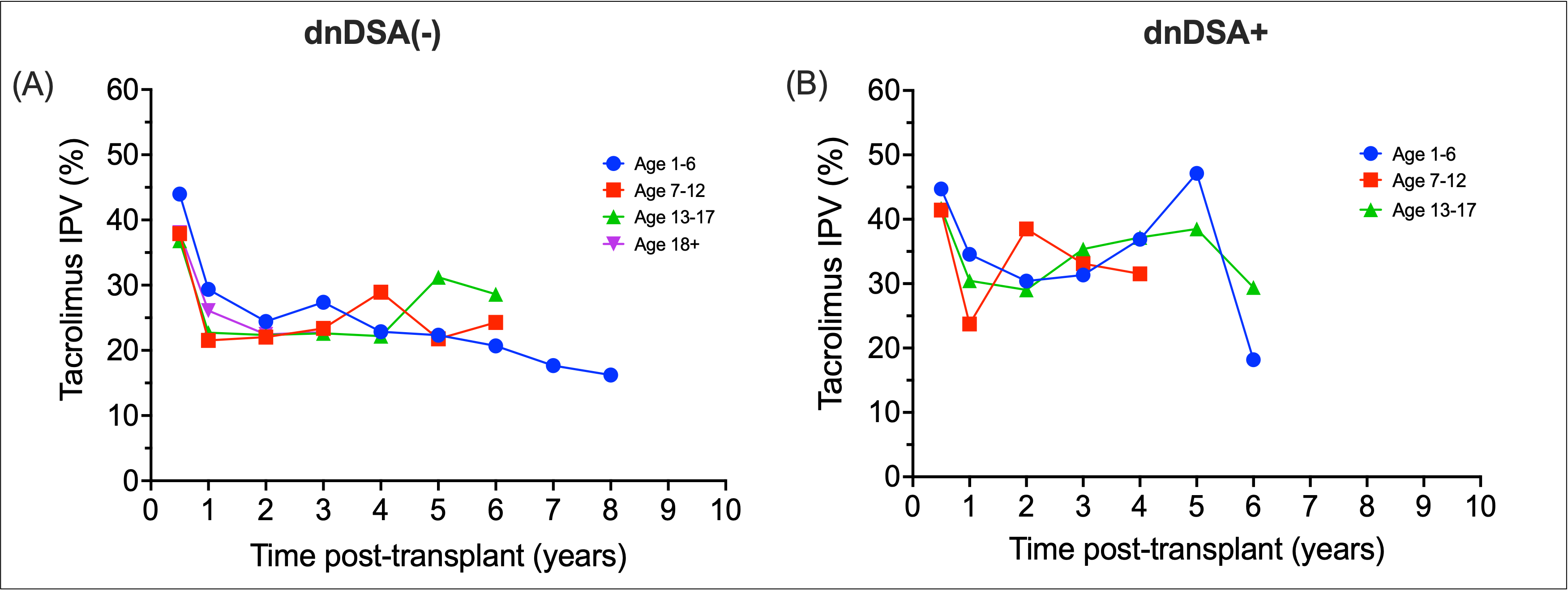
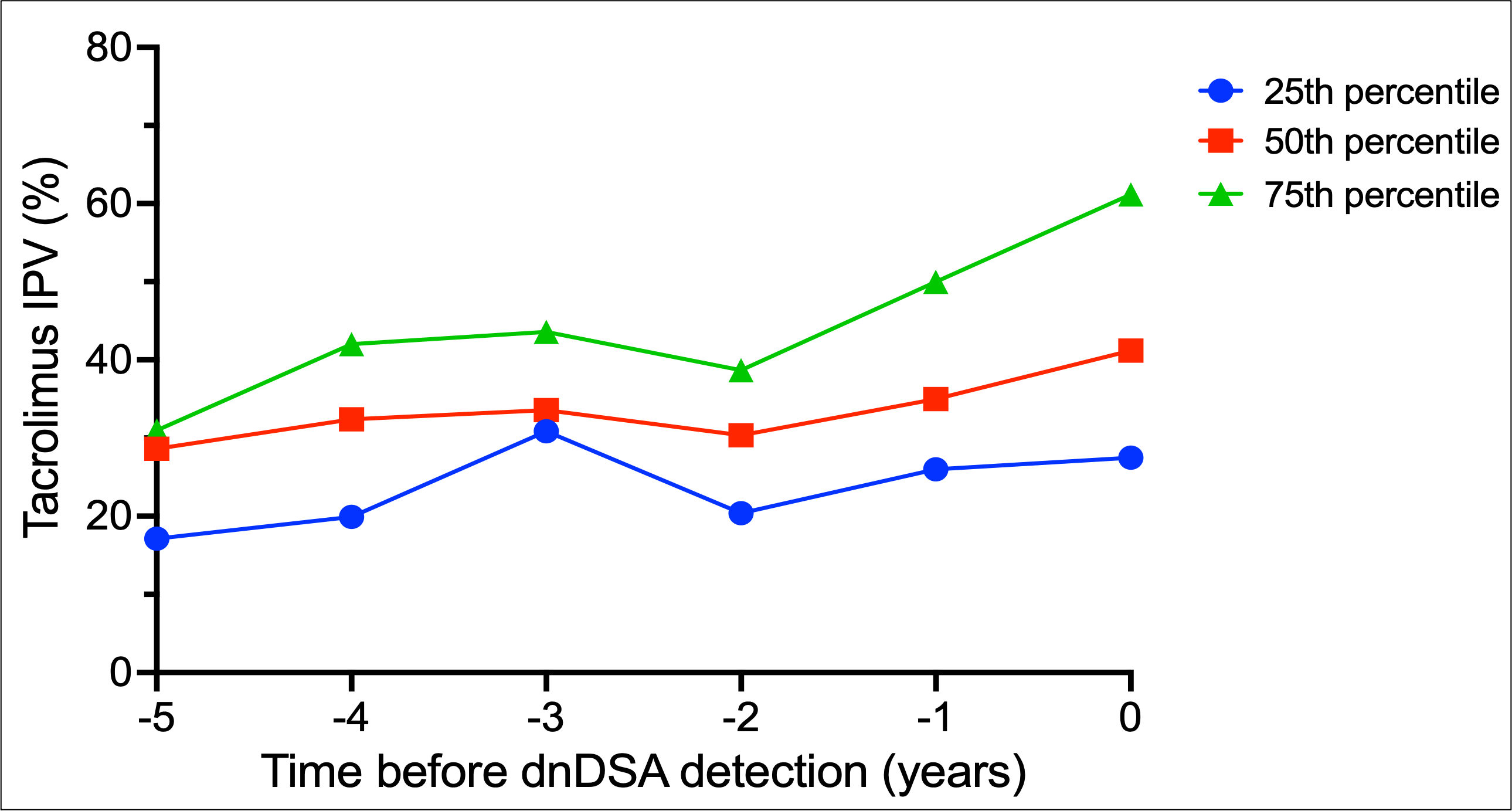
*Results: 220 patients were included, with a combined 14954 tacrolimus levels (median of 58 levels per patient). Median age was 12.8 years, 55.2% were male, and 24.5% had living donor transplants. Median follow-up time was 44.6 months. 51 patients formed dnDSA. 14 patients had graft loss. Median tacrolimus IPV for the cohort was 30.6%, with 51.6% of patients exceeding 30% variability. Patients who formed dnDSA had greater fluctuations in tacrolimus IPV over time, compared to non-DSA formers [Figure 1]. In dnDSA+ patients, a rise in median tacrolimus IPV was noted prior to dnDSA detection [Figure 2]. Tacrolimus IPV >30% was found to be significantly associated with dnDSA formation in both unadjusted (HR 4.97, 95% CI 2.31 – 10.72) and adjusted models (HR 5.30, 95% CI 2.43 – 11.57).
*Conclusions: Tacrolimus IPV trends differ between age groups. Trends in increasing IPV were noted in patients that formed dnDSA years prior to initial detection. High IPV was associated with dnDSA formation. Patterns of IPV may identify patients at increased risk of adverse graft outcomes.
Figure 1.
Figure 2.
To cite this abstract in AMA style:
Piburn K, Maestretti L, Patton MV, McGrath AM, Indridason OS, Chaudhuri A, Grimm P, Sigurjonsdottir V. Baseline Trends in Tacrolimus Intrapatient Variability in Pediatric and Young Adult Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/baseline-trends-in-tacrolimus-intrapatient-variability-in-pediatric-and-young-adult-kidney-transplant-recipients/. Accessed February 28, 2026.« Back to 2022 American Transplant Congress