Avoidance of CNI and Steroids Using Belatacept – A Preliminary Report of CTOT-16.
1UAB, Birmingham
2UCSF, San Francisco
3Emory, Atlanta
4Rho, Chapel Hill
5NIAID, Bethesda.
Meeting: 2016 American Transplant Congress
Abstract number: 566
Keywords: Co-stimulation, Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Time: 8:30am-10:00am
 Presentation Time: 8:30am-8:45am
Presentation Time: 8:30am-8:45am
Location: Veterans Auditorium
CTOT-16 tested the hypothesis that belatacept used as maintenance immunosuppression (IS) in kidney transplantation would allow the long-term avoidance of CNI and corticosteroids. Methods: Primary renal transplant recipients who were EBV seropositive and without DSA or a positive CXM, were randomized to 3 groups. IS regimens for each group are depicted in Table I. 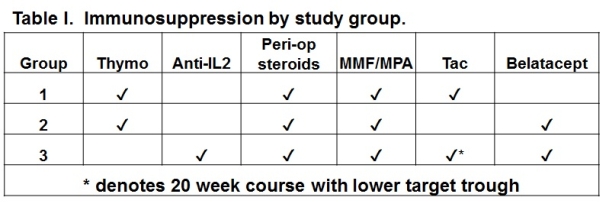 Endpoints included eGFR, the incidence and severity of rejection, and safety measures. Results: Due to concerns about the increased rates of acute rejection (AR) enrollment in group 3 was halted in August 2014 and the entire study was stopped in April 2015 after 69 patients were randomized. Groups were comparable with respect to demographics. The number of patients enrolled in each group and selected outcome measures are shown in Table II.
Endpoints included eGFR, the incidence and severity of rejection, and safety measures. Results: Due to concerns about the increased rates of acute rejection (AR) enrollment in group 3 was halted in August 2014 and the entire study was stopped in April 2015 after 69 patients were randomized. Groups were comparable with respect to demographics. The number of patients enrolled in each group and selected outcome measures are shown in Table II. 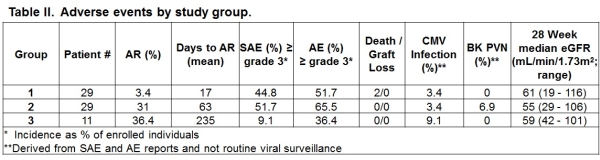 As noted rejection rates were increased in the groups receiving CNI-free belatacept-based long-term IS regimens. No Banff grade II or III rejections occurred in groups 1 or 3 while group 2 had 4 grade IIA and 2 grade III rejections. Recurrent episodes of AR were seen in 2 patients (1 each in group 1 and 2). Two subjects experienced AMR (one each in groups 1 and 2). Conclusion: The increased rates of AR observed in groups 2 and 3 suggest that belatacept may not provide adequate immunosuppression to allow avoidance of CNI (at least at relatively early time points) and corticosteroids. Additional analyses are ongoing in an attempt to identify clinical or laboratory factors that may identify high or low risk groups for treatment with a belatacept-based CNI-free regimen as well as assessment of the long-term impact of CNI avoidance on renal function and cardiovascular and metabolic risk profiles.
As noted rejection rates were increased in the groups receiving CNI-free belatacept-based long-term IS regimens. No Banff grade II or III rejections occurred in groups 1 or 3 while group 2 had 4 grade IIA and 2 grade III rejections. Recurrent episodes of AR were seen in 2 patients (1 each in group 1 and 2). Two subjects experienced AMR (one each in groups 1 and 2). Conclusion: The increased rates of AR observed in groups 2 and 3 suggest that belatacept may not provide adequate immunosuppression to allow avoidance of CNI (at least at relatively early time points) and corticosteroids. Additional analyses are ongoing in an attempt to identify clinical or laboratory factors that may identify high or low risk groups for treatment with a belatacept-based CNI-free regimen as well as assessment of the long-term impact of CNI avoidance on renal function and cardiovascular and metabolic risk profiles.
CITATION INFORMATION: Mannon R, Stock P, Mehta A, Ikle D, Watson N, Bridges N, Robien M, Newell K. Avoidance of CNI and Steroids Using Belatacept – A Preliminary Report of CTOT-16. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Mannon R, Stock P, Mehta A, Ikle D, Watson N, Bridges N, Robien M, Newell K. Avoidance of CNI and Steroids Using Belatacept – A Preliminary Report of CTOT-16. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/avoidance-of-cni-and-steroids-using-belatacept-a-preliminary-report-of-ctot-16/. Accessed February 18, 2026.« Back to 2016 American Transplant Congress
