Associations Between Sirolimus Use and Outcomes After Liver Transplant for Hepatocellular Carcinoma
1Natl. Cancer Inst., Bethesda, MD
2U. of Minnesota, Minneapolis, MN
3Sci. Reg. of Transplant Recipients, Minneapolis, MN.
Meeting: 2015 American Transplant Congress
Abstract number: D180
Keywords: Hepatocellular carcinoma, Mortality, Rapamycin, Recurrence
Session Information
Session Name: Poster Session D: Liver Transplantation for Hepatocellular Carcinoma
Session Type: Poster Session
Date: Tuesday, May 5, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
A growing fraction of U.S. liver transplants are being done for the indication of hepatocellular carcinoma (HCC). HCC recurrence after transplant remains a major concern in this population. Use of sirolimus, an immunosuppressant and mTOR inhibitor with anticarcinogenic properties, may reduce HCC recurrence and improve overall survival. The U.S. Scientific Registry of Transplant Recipients (SRTR) was linked to national pharmacy claims to confirm sirolimus use among recipients of liver transplants for HCC. Recipients with sirolimus in their SRTR discharge regimen and ≥1 sirolimus claim in the first 3 months post-transplant were included as sirolimus users. Those with no sirolimus in their discharge regimen and non-sirolimus immunosuppressant claims in the first 3 months were included as sirolimus non-users. Cox regression was used to estimate associations with HCC recurrence and all-cause mortality adjusting for sex, race, ethnicity, age, calendar year, alpha-fetoprotein level, hepatitis C, and total tumor volume at transplant. We included 4,181 liver transplants for HCC, of which 240 (6%) were sirolimus users. Fewer sirolimus users had hepatitis C before transplant when compared to sirolimus non-users, but other recipient characteristics were similar.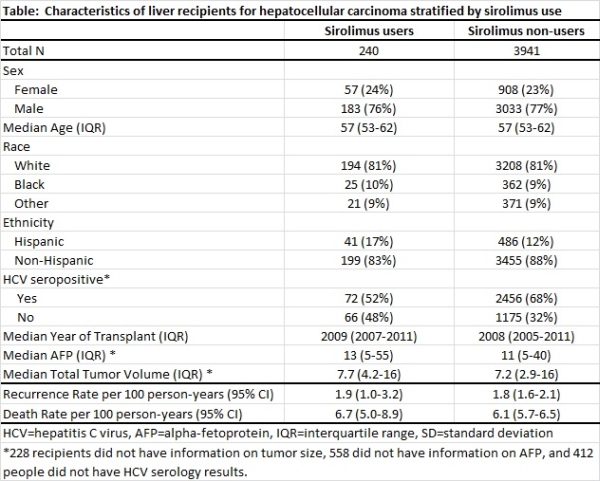 HCC recurrence rates for sirolimus users and non-users were similar (1.9 and 1.8 per 100 person-years, respectively) and did not differ after adjustment for recipient characteristics (hazard ratio [HR]=0.8, 95%CI=0.4-1.6). All-cause mortality was also similar for sirolimus users and non-users (6.7 and 6.1 deaths per 100 person-years, respectively), even after adjustment for recipient characteristics (HR=1.1, 95%CI=0.8-1.5). Sirolimus use was not associated with lower rates of HCC recurrence or mortality. Recipients more vulnerable to recurrence could have been disproportionately prescribed sirolimus, but the recipient characteristics we examined were similar. Our results suggest sirolimus use is not beneficial compared to other immunosuppressant options in this population.
HCC recurrence rates for sirolimus users and non-users were similar (1.9 and 1.8 per 100 person-years, respectively) and did not differ after adjustment for recipient characteristics (hazard ratio [HR]=0.8, 95%CI=0.4-1.6). All-cause mortality was also similar for sirolimus users and non-users (6.7 and 6.1 deaths per 100 person-years, respectively), even after adjustment for recipient characteristics (HR=1.1, 95%CI=0.8-1.5). Sirolimus use was not associated with lower rates of HCC recurrence or mortality. Recipients more vulnerable to recurrence could have been disproportionately prescribed sirolimus, but the recipient characteristics we examined were similar. Our results suggest sirolimus use is not beneficial compared to other immunosuppressant options in this population.
To cite this abstract in AMA style:
Yanik E, Chinnakotla S, Israni A, Snyder J, Gustafson S, Engels E. Associations Between Sirolimus Use and Outcomes After Liver Transplant for Hepatocellular Carcinoma [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/associations-between-sirolimus-use-and-outcomes-after-liver-transplant-for-hepatocellular-carcinoma/. Accessed February 25, 2026.« Back to 2015 American Transplant Congress
