Are Tacrolimus Concentrations Reduced with Direct-acting Antiviral Administration in Transplant Recipients?
K. Huang, K. Farrow, M. Christian
Pharmacy, Penn State Health Hershey Medical Center, Hershey, PA
Meeting: 2021 American Transplant Congress
Abstract number: 361
Keywords: Drug interaction, Hepatitis C, Monitoring, N/A
Topic: Clinical Science » Infectious Disease » Non-Organ Specific: Viral Hepatitis
Session Information
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:15pm-6:20pm
Presentation Time: 6:15pm-6:20pm
Location: Virtual
*Purpose: To evaluate the effects of direct-acting antivirals (DAAs) on tacrolimus trough concentrations and clinical outcome and to assess the need for a priori dose adjustments
*Methods: In this single-center retrospective chart review, 164 liver, kidney, and heart transplant recipients with a diagnosis of hepatitis C virus (HCV) infection between October 1, 2014 and January 1, 2020 were screened. Those who were 18 years of age or older and starting DAA therapy with concomitant tacrolimus post-transplant were included. Patients were excluded if they were on concurrent moderate or strong CYP3A4 inhibitors or inducers; failed to complete DAA therapy; had HCV cure prior to transplant; or had missing labs at any of the following time points, including baseline, week 4 of DAA therapy, end of therapy, or sustained virologic response (SVR) at 12 weeks. The primary outcome was the percent change in tacrolimus trough concentrations at SVR12 compared to baseline. Tacrolimus doses and trough concentrations were examined at all above time points, and incidences of acute rejection, graft loss, and mortality were also reviewed.
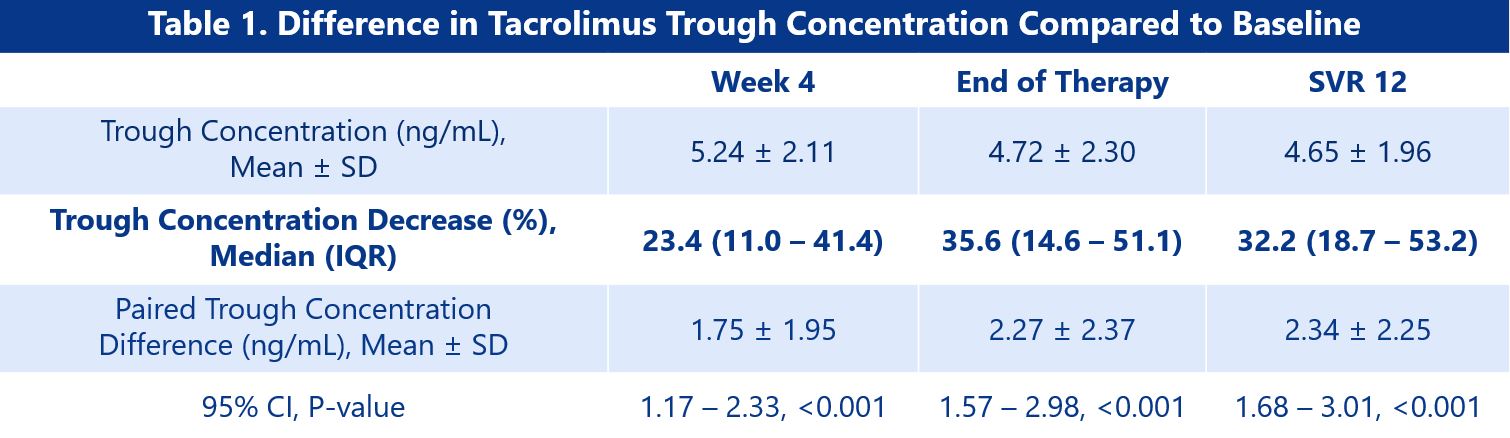
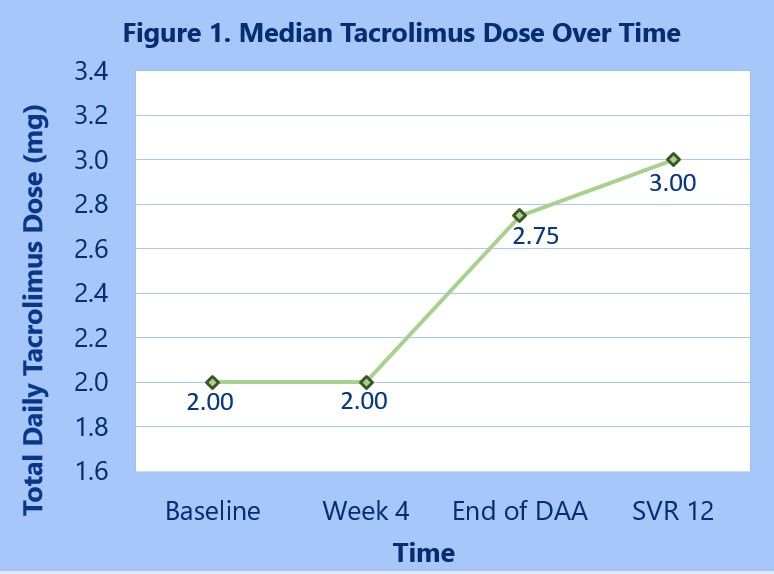
*Results: 46 patients were included in the final analysis, and regardless of DAA agent used, trough concentrations decreased by a median of 32.2% from an average of 6.99 ± 2.07 ng/mL at baseline to 4.65 ± 1.96 ng/mL at SVR12 (p < 0.001). Results of trough concentration differences are summarized in Table 1. Median total daily tacrolimus doses increased from 2.0 mg/day prior to DAA initiation to 3.0 mg/day at SVR12 (Figure 1). 15 patients (32.6%) required dose increases with DAA treatment, and two patients experienced an episode of rejection within this timeframe.
*Conclusions: The treatment of HCV infection with DAAs leads to statistically significant reductions in tacrolimus trough concentrations. This is likely due to the improvement in liver enzyme function and metabolism, which seems to supersede anticipated DAA-tacrolimus drug-drug interactions. The clinical significance of these subtherapeutic changes are yet to be determined, but for patients on concurrent tacrolimus and DAA therapy, tacrolimus must be monitored closely, and empiric dose increases should be considered.
To cite this abstract in AMA style:
Huang K, Farrow K, Christian M. Are Tacrolimus Concentrations Reduced with Direct-acting Antiviral Administration in Transplant Recipients? [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/are-tacrolimus-concentrations-reduced-with-direct-acting-antiviral-administration-in-transplant-recipients/. Accessed February 24, 2026.« Back to 2021 American Transplant Congress