A Search for a Biomarker Which Predicts Belatacept-Resistant Rejection.
1Internal Medicine, Erasmus University Medical Center, Rotterdam, South Holland, Netherlands
2Pathology, Erasmus University Medical Center, Rotterdam, South Holland, Netherlands
3Immunohematology and Blood Transfusion, Erasmus University Medical Center, Rotterdam, South Holland, Netherlands
4Biostatistics, Erasmus University Medical Center, Rotterdam, South Holland, Netherlands
Meeting: 2017 American Transplant Congress
Abstract number: 132
Keywords: Immunosuppression, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney: Acute Cellular Rejection
Session Type: Concurrent Session
Date: Sunday, April 30, 2017
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: E451b
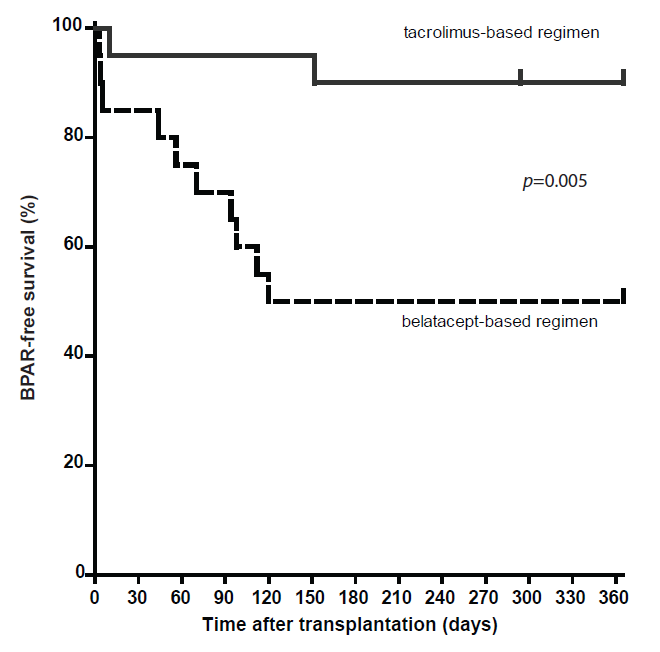
Belatacept, an inhibitor of the CD28-CD80/86 costimulatory pathway, has been associated with a higher acute rejection risk than ciclosporin. We sought to identify a biomarker for belatacept-resistant rejection.
In this randomized controlled trial, 40 kidney transplant recipients were 1:1 randomized to belatacept or tacrolimus combined with basiliximab, mycophenolate mofetil and prednisolone. The 1-year incidence of biopsy-proven acute rejection (BPAR) was monitored. Potential biomarkers, namely CD8+CD28–, CD4+CD57+PD1– and CD8+CD28++ EMRA T-cells were measured pre-transplantation and correlated to BPAR after transplantation. Pharmacodynamic monitoring of belatacept was performed by measuring free CD86 on circulating monocytes.
The incidence of BPAR was higher among belatacept-treated than tacrolimus-treated patients: 50% vs. 10%; p = 0.01. The majority of rejections occurred within 3 months after transplantation. Three graft losses, due to BPAR, occurred in the belatacept group vs. none in the tacrolimus group. There were no differences in pre-transplant values of the biomarkers between rejectors and non-rejectors in the belatacept group. In univariable Cox regression analyses, the studied cell subsets were not significantly associated with the risk of developing BPAR. CD86 molecules on circulating monocytes in belatacept-treated patients were saturated at all time points.
Belatacept-based immunosuppressive therapy resulted in significantly higher and more severe acute rejection compared to standard, tacrolimus-based therapy. Neither cellular biomarkers nor insufficient blockade of the CD28-CD80/86 co-stimulatory pathway predicted BPAR.
CITATION INFORMATION: de Graav G, Baan C, Clahsen-van Groningen M, Kraaijeveld R, Dieterich M, Verschoor W, Roelen D, Cadogan M, van de Wetering J, van Rosmalen J, Weimar W, Hesselink D. A Search for a Biomarker Which Predicts Belatacept-Resistant Rejection. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Graav Gde, Baan C, Groningen MClahsen-van, Kraaijeveld R, Dieterich M, Verschoor W, Roelen D, Cadogan M, Wetering Jvande, Rosmalen Jvan, Weimar W, Hesselink D. A Search for a Biomarker Which Predicts Belatacept-Resistant Rejection. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-search-for-a-biomarker-which-predicts-belatacept-resistant-rejection/. Accessed February 18, 2026.« Back to 2017 American Transplant Congress