A Pilot Study of Mkidney: A Novel Mobile Health Platform to Support OPTN Living Donor Follow-Up Data Collection
1Johns Hopkins School of Medicine, Baltimore, MD, 2The Texas Transplant Institute, Methodist Specialty and Transplant Hospital, San Antonio, TX, 3Vanderbilt University Medical Center, Nashville, TN
Meeting: 2020 American Transplant Congress
Abstract number: 561
Keywords: Donation, Kidney, Living donor, Patient education
Session Information
Session Name: Kidney Living Donor: Other II
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
*Purpose: Transplant hospitals struggle to meet Organ Procurement and Transplantation Network (OPTN) requirements for LKD follow-up. In the face of barriers such as cost, LKD inconvenience, and the burden of data collection, they lack the tools to improve LKD engagement. To address this critical health system failure, we built the mKidney® System based on input elicited from LKDs, transplant providers, and thought leaders in the field of transplantation.
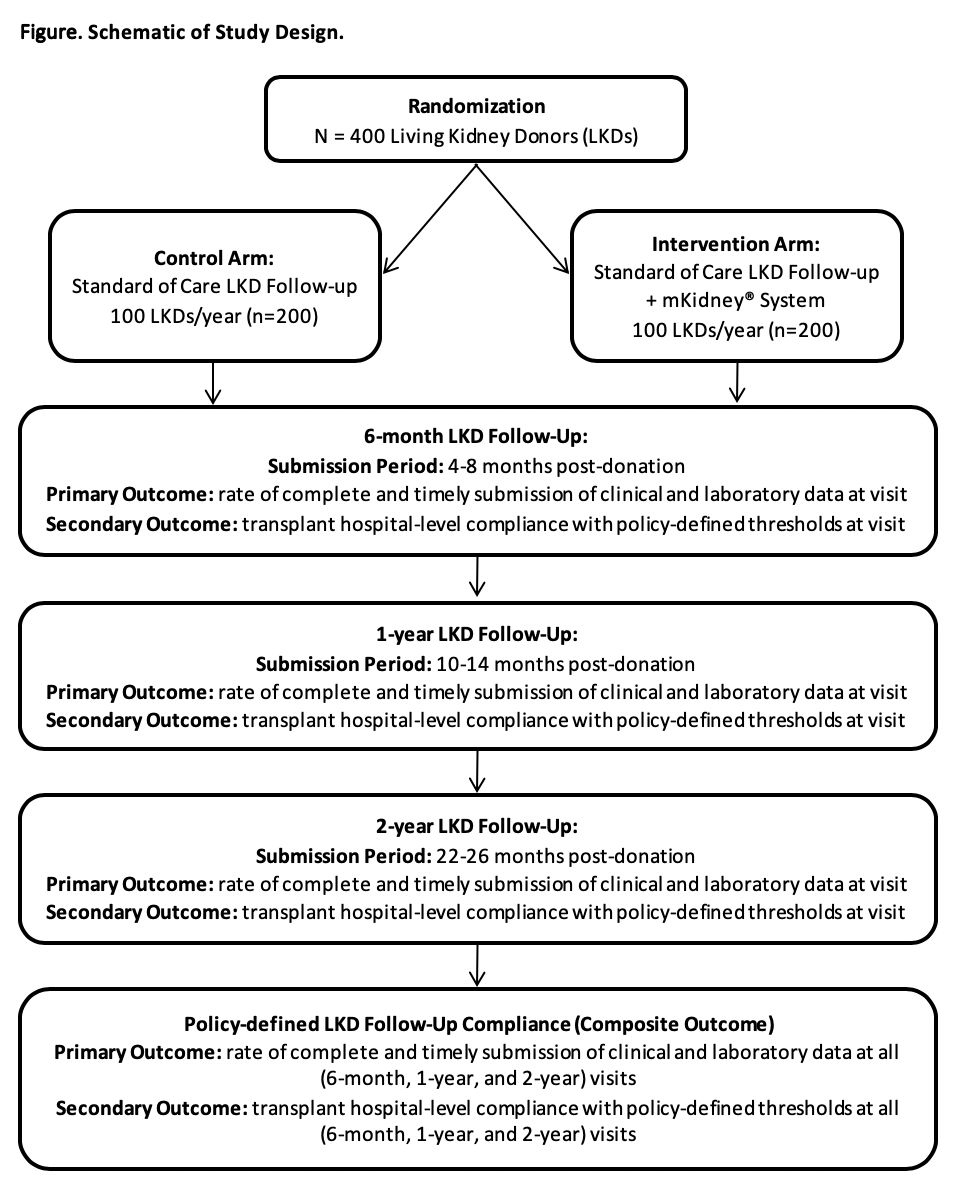
*Methods: Key features of the mKidney® System include a HIPAA-compliant patient-facing smartphone app and transplant provider-facing web portal, automated short message service (SMS) text messages, email, and push notifications, and data export functionality. We are conducting an ongoing randomized controlled trial (RCT) to evaluate the impact of the mKidney® System on rates of post-donation follow-up, in preparation for a fully-powered multi-site clinical trial (NCT03400085). Participants are randomized to the intervention (mKidney® System) or control arm (standard of care) using block randomization, and follow-up compliance is tracked over time (Figure). Follow-up compliance is ascertained through linkage to national registry data (SRTR).
*Results: To date, our pilot study population includes 237 participants; of whom, 101 have 6-month donor follow-up available from the SRTR registry. Clinical follow-up required for compliance was completed by 46/46 (100%) of participants in the mKidney® arm vs 53/55 (96.4%) of participants in the control arm (Fisher exact p = .5). Required lab follow-up was completed by 45/46 (97.8%) of participants in the mKidney® arm vs 46/55 participants (83.6%) in the control arm (Fisher exact p=0.02).
*Conclusions: Designed specifically to facilitate the collection and reporting of LKD follow-up data, the mKidney® System is a promising new solution to improve historically poor care management for LKDs in the US.
To cite this abstract in AMA style:
Sidoti C, Zhang W, Waldram M, Thomas A, Levan M, Massie A, Bingaman A, Forbes R, Edwards G, Warmke K, Centanni K, Segev D, Henderson M. A Pilot Study of Mkidney: A Novel Mobile Health Platform to Support OPTN Living Donor Follow-Up Data Collection [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/a-pilot-study-of-mkidney-a-novel-mobile-health-platform-to-support-optn-living-donor-follow-up-data-collection/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress