A Novel Endpoint for Evaluating the Effect of Everolimus with Reduced Calcineurin Inhibitor on Long-Term Kidney Graft Outcome: The TRANSFORM Study Protocol.
1TRANSFORM Study Group, Murray
2Novartis Pharma AG, Basel, Switzerland
Meeting: 2017 American Transplant Congress
Abstract number: D95
Keywords: Glomerular filtration rate (GFR), Kidney transplantation, Rejection
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Purpose: With the advancement of immunosuppressive (IS) agents, while short-term graft outcomes improved significantly, long-term outcomes remain a concern. Both acute rejection (AR) and nephrotoxicity are modifiable by type of IS regimen. Therefore, there is a need for a meaningful endpoint that can predict long-term benefit of IS regimens by integrating short term anti-rejection efficacy (AR) and short-term renal function level.
Method/Results: Published literature was thoroughly reviewed to identify factors influencing rates of AR and estimated glomerular filtration (eGFR; at 12 Month [M]) associated to risk for long-term death-censored graft failure. With regard to AR, number of episodes, late occurrence (>3M post-transplant [Tx]), severity, need for antibody treatment and recovery of renal function after rejection have influence on long-term graft survival. Due to current low rates of AR and 5-years post-Tx survival with a functioning graft of 80%, a large study population is needed to demonstrate significant differences in efficacy of IS regimen. Regarding graft function, an eGFR <50 mL/min/1.73 m2 has consistently been associated with poor long-term graft survival (Table 1). The ongoing 24M, multicenter, open-label, randomized TRANSFORM (NCT01950819) study, uses the novel composite endpoint of anti-rejection efficacy (treated biopsy proven AR [tBPAR]) or graft function at 12M (eGFR <50 mL/min/1.73 m2) to better indicate the effect of everolimus with reduced CNI exposure versus mycophenolate with standard CNI exposure on long-term graft outcome in a large population of >2000 patients.
Conclusion: An endpoint that integrates renal function and freedom from rejection is an unmet need. TRANSFORM offers the opportunity to test a novel endpoint in the context of the largest study ever done in kidney transplantation addressing this unmet need.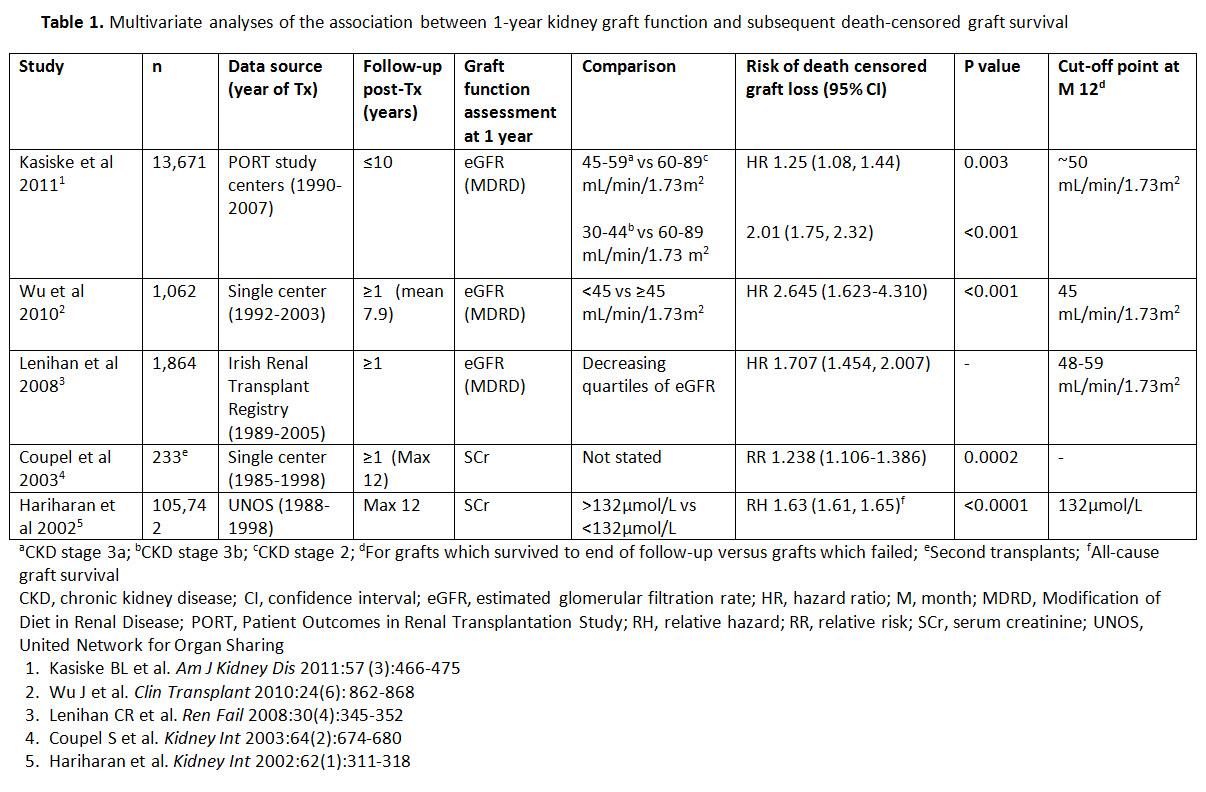
CITATION INFORMATION: Srinivas T, Legendre C, Oppenheimer F, Tedesco-Silva H, Luo W.-L, Bernhardt P, Sommerer C, Chadban S, Vincenti F, Pascual J. A Novel Endpoint for Evaluating the Effect of Everolimus with Reduced Calcineurin Inhibitor on Long-Term Kidney Graft Outcome: The TRANSFORM Study Protocol. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Srinivas T, Legendre C, Oppenheimer F, Tedesco-Silva H, Luo W-L, Bernhardt P, Sommerer C, Chadban S, Vincenti F, Pascual J. A Novel Endpoint for Evaluating the Effect of Everolimus with Reduced Calcineurin Inhibitor on Long-Term Kidney Graft Outcome: The TRANSFORM Study Protocol. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/a-novel-endpoint-for-evaluating-the-effect-of-everolimus-with-reduced-calcineurin-inhibitor-on-long-term-kidney-graft-outcome-the-transform-study-protocol/. Accessed March 3, 2026.« Back to 2017 American Transplant Congress
