A Follow-Up Report on the Use of IVIG+Rituximab to Reduce Donor Specific Antibodies and Improve Transplant Rates in Highly-Sensitized Patients Awaiting Deceased Donor Kidney Transplantation
Medicine/Nephrology, Cedars Sinai Medical Center, Los Angeles, CA.
Meeting: 2018 American Transplant Congress
Abstract number: 323
Keywords: Antibodies, Highly-sensitized, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Desensitization
Session Type: Concurrent Session
Date: Monday, June 4, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 4B
Introduction: Desensitization (DES) with IVIG/rituximab is associated with a reduction in donor specific antibodies (DSA), improved transplant rates and outcomes in highly-sensitized (HS) patients. We report 36 month results of a prospective clinical trial evaluating the efficacy of rituximab in lowering DSA and reducing allograft injury (NCT01178216; IND 109067).
Methods: From 2013-16, 39 HS patients with cPRA≥50% underwent DES with IVIG 2 g/kg on day 0 and 30 and rituximab 1g on day 15 while awaiting kidney transplant. Patients transplanted within 9M of DES received another rituximab 1g at 3 and/or 6M if DSA was present and underwent protocol biopsy at 12M. The primary endpoint was the rate of transplant glomerulopathy (TG). Patient/graft survival, freedom from ABMR±TG, presence of DSA, and eGFR up to 36M were also assessed.
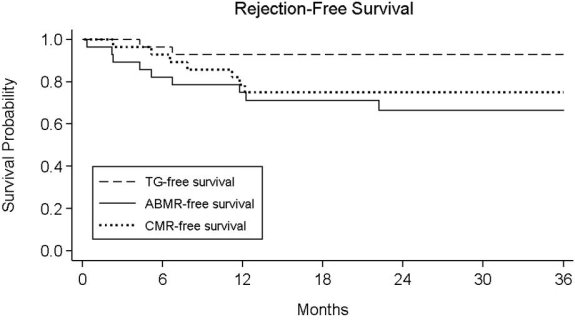
Results: Twenty-nine patients (74.4%) received deceased donor transplants at a mean 7.6±6.1M after DES. Of these, 24 (82.7%) were DSA+, FCXM+, or both. One/5/0 had de novo/persistent/rebound DSA at 3M vs. 1/3/0 at 6M. Sixteen (55%) were transplanted within 9M of DES and participated in the post-transplant study protocol, receiving another dose of rituximab at 3M (5/16, 31.3%) and/or 6M (3/16, 18.8%) if DSA was detected. Two (6.9%) overall developed TG (36M TG-free survival: 92.5%; fig. 1); only 1 patient in the post-transplant study protocol developed TG (36M TG-free survival: 93.3%). Death-censored graft survival was 84.9% and patient survival was 94.4% at 36M. At last follow-up, 8/29 patients (28%) had ongoing DSA. Mean eGFR was stable over the study period (12M: 64.7 ± 23.5; 24M: 69.5 ± 18.9; 36M: 69.8 ± 14.3 ml/min/1.73 m2).
Conclusions: Pre-transplant DES with IVIG/rituximab facilitated DD in 74% of participants, the majority of whom were HLA-incompatible. Post-transplant DSA monitoring with rituximab at 3 and/or 6M for persistent DSA was associated with a low rate of TG, stable eGFR, and excellent patient/graft survival up to 36M.
CITATION INFORMATION: Najjar R., Huang E., Vo A., Choi J., Lim K., Louie S., Peng A., Sethi S., Toyoda M., Zhang X., Jordan S. A Follow-Up Report on the Use of IVIG+Rituximab to Reduce Donor Specific Antibodies and Improve Transplant Rates in Highly-Sensitized Patients Awaiting Deceased Donor Kidney Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Najjar R, Huang E, Vo A, Choi J, Lim K, Louie S, Peng A, Sethi S, Toyoda M, Zhang X, Jordan S. A Follow-Up Report on the Use of IVIG+Rituximab to Reduce Donor Specific Antibodies and Improve Transplant Rates in Highly-Sensitized Patients Awaiting Deceased Donor Kidney Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/a-follow-up-report-on-the-use-of-ivigrituximab-to-reduce-donor-specific-antibodies-and-improve-transplant-rates-in-highly-sensitized-patients-awaiting-deceased-donor-kidney-transplantation/. Accessed February 22, 2026.« Back to 2018 American Transplant Congress