1-Year Safety and Efficacy of Eculizumab in Adult aHUS Patients, With or Without a History of Renal Transplant
1University Paris Descartes & Hôpital Necker, Paris, France
2Alexion, Cheshire, CT
3Assistance Publique–Hôpitaux Paris, Hôpital Robert-Debré, Paris, France.
Meeting: 2015 American Transplant Congress
Abstract number: 361
Keywords: Glomerular filtration rate (GFR), Hemolytic-uremic syndrome, Kidney transplantation, Renal failure
Session Information
Session Name: Concurrent Session: Glomerulonephritis/Recurrent Disease
Session Type: Concurrent Session
Date: Tuesday, May 5, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Room 121-AB
Background: Atypical hemolytic-uremic syndrome (aHUS) is a life-threatening, chronic disease of complement-mediated TMA. In aHUS, renal transplantation is associated with post-transplant (TP) TMA and ∼50–70% graft loss after 5 years. Eculizumab (ECU) inhibits complement-mediated TMA and was shown to be safe and efficacious regardless of TP history in the largest prospective study of aHUS patients (pts), and the first in an adult population.
Methods: A retrospective subanalysis of an open-label, single-arm trial of ECU in pts ≥18 years with native and transplanted kidneys was performed. Patients were vaccinated against N. meningitidis. Inclusion criteria included platelet count <150×109/L, lactate dehydrogenase ≥1.5xULN, and serum creatinine ≥ULN. Primary outcomes were evaluated at week 26; this analysis assessed outcomes after 1 year.
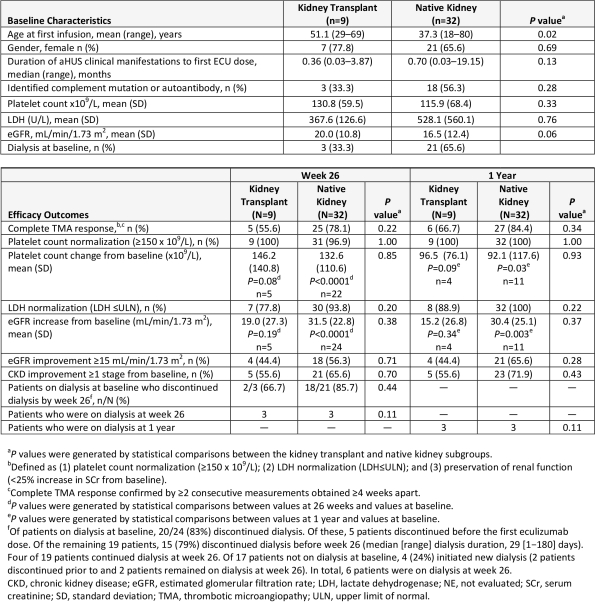
Results: There were 9 (22%) TP pts (6 with post-TP aHUS recurrence [following 2 TP in 1 pt, and 1 TP in 5 pts] and 3 with de novo post-TP aHUS) and 32 with native kidneys. Improvements in hematologic and renal parameters occurred in TP and native-kidney pts (Table). At 1 year, mean change from baseline in platelet count was 96.5 (P=0.09) and 92.1 x 109/L (P=0.03), and in eGFR was 15.2 (P=0.34) and 30.4 mL/min/1.73 m2 (P=0.003), respectively. Of pts on baseline dialysis, 2/3 (66.7%) TP and 18/21 (85.7%) with native kidneys discontinued dialysis by week 26. Six pts were on dialysis at week 26 and 1 year. Treatment-emergent adverse events were similar among subgroups. Two pts had meningococcal infections before week 26; both recovered and 1 continued ECU.
Conclusions: Ongoing ECU continued to inhibit complement-mediated TMA and produced clinically meaningful improvements in platelet count and renal function after 1 year, regardless of TP history. No pt progressed to ESRD requiring TP; most remained dialysis-free. These results are consistent with subanalyses from previous trials, in which ECU prevented graft loss and improved renal function in aHUS pts with TP and native kidneys.
To cite this abstract in AMA style:
Legendre C, Kincaid J, Bedrosian C, Loirat C. 1-Year Safety and Efficacy of Eculizumab in Adult aHUS Patients, With or Without a History of Renal Transplant [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/1-year-safety-and-efficacy-of-eculizumab-in-adult-ahus-patients-with-or-without-a-history-of-renal-transplant/. Accessed March 9, 2026.« Back to 2015 American Transplant Congress
