Renal Outcome with Eculizumab in Patients with aHUS with Native and Transplanted Kidneys: Pooled Analysis of 100 Patients.
1Université
Paris Descartes & Hôpital Necker, Paris, France
2Hospital Clinic, University of Barcelona, Barcelona, Spain
3University Hospital Schleswig-Holstein, Kiel, Germany
4Alexion Pharmaceuticals, Inc., Cheshire, CT
5IRCCS-Istituto di Ricerche Farmacologiche Mario Negri, Bergamo, Italy
6CHU de Liège, Liège, Belgium
7Newcastle University, Newcastle upon Tyne, United Kingdom.
Meeting: 2016 American Transplant Congress
Abstract number: 402
Keywords: Glomerular filtration rate (GFR), Hemolytic-uremic syndrome, Kidney transplantation, Renal failure
Session Information
Session Name: Concurrent Session: It's Just Not the Donor: Impact of Recipient Factors on Outcomes
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:18pm-3:30pm
Presentation Time: 3:18pm-3:30pm
Location: Ballroom A
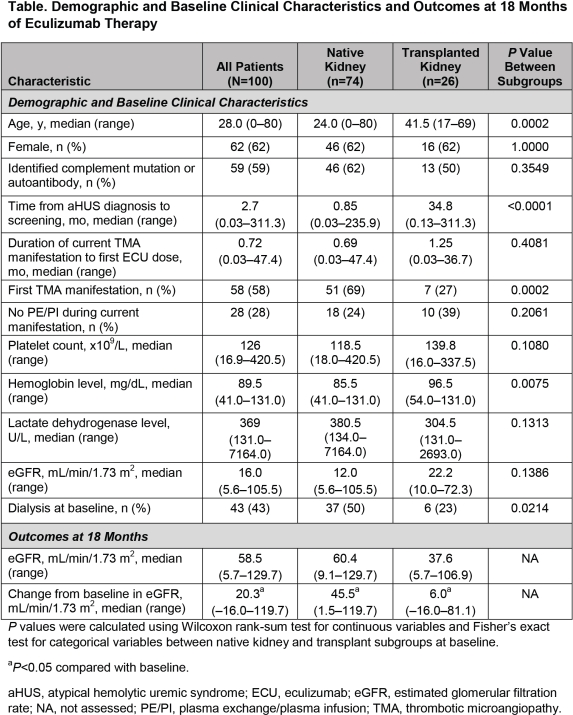
Purpose: aHUS often progresses to ESRD and requires transplant (tx). Eculiumab (ECU), a terminal complement inhibitor, inhibits TMA irrespective of tx status. We evaluated outcomes in patients (pts) with aHUS and native and tx kidneys treated with ECU.
Methods: Efficacy and safety data over 18 months from 4 phase 2 trials of ECU with long-term extensions were analyzed post hoc.
Results: Data from 100 pts (74 with native kidneys; 26 with 38 tx prebaseline) were pooled. At baseline, extent of renal injury (ie, age, time from diagnosis to treatment, and TMA history) differed (P≤0.0002; Table). No grafts were lost on ECU. One pt in ESRD for >4 months prebaseline was txed after ECU initiation. Median (range) ECU duration was 71 (0–186) weeks. Median (range) eGFR increased from baseline to 18 months to 60 (9-130) and 38 (6-107) mL/min/1.73 m2 for pts with native and tx kidneys (Table). ECU was well tolerated; most adverse events were mild or moderate. Meningococcal infections occurred in 2 pts (1 native kidney, serogroup B; 1 with tx, serogroup unknown). Both were vaccinated; neither received prophylactic antibiotics at time of infection. Infections resolved with antibiotics; the pt with the native kidney continued ECU.
Conclusion: Graft loss occurs at a high rate in txed pts with aHUS prior to ECU. ECU was well tolerated and improved renal function in pts with aHUS with native and txed kidneys. No pts with prebaseline tx lost grafts during ECU treatment. Extent of previous renal injury influences changes in eGFR in pts with aHUS (Nephrol Dial Transplant. 2015;30[suppl 3]:SaO008), particularly those with tx (Am J Transplant. 2012;12:3337–54). These data suggest that early recognition and treatment of aHUS optimizes renal outcomes.
CITATION INFORMATION: Legendre C, Campistol J, Feldkamp T, Wang J, Remuzzi G, Weekers L, Sheerin N. Renal Outcome with Eculizumab in Patients with aHUS with Native and Transplanted Kidneys: Pooled Analysis of 100 Patients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Legendre C, Campistol J, Feldkamp T, Wang J, Remuzzi G, Weekers L, Sheerin N. Renal Outcome with Eculizumab in Patients with aHUS with Native and Transplanted Kidneys: Pooled Analysis of 100 Patients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/renal-outcome-with-eculizumab-in-patients-with-ahus-with-native-and-transplanted-kidneys-pooled-analysis-of-100-patients/. Accessed February 11, 2026.« Back to 2016 American Transplant Congress
