Likelihood of Improving or Maintaining Renal Function in Recipients of Extended-Criteria Donor Kidneys over Five Years with Belatacept or CsA (BENEFIT-EXT Long-Term Extension Study)
Univ Hosp Bellvitge, Spain
Univ Hosp Gasthuisberg, Belgium
Bicetre Hosp, France
Inst de Nefrologia, Argentina
Univ of Minnesota, US
Medizinische Hoschschule, Germany
Bristol-Myers Squibb, US
Hosp do Rim e Hipertensao, Brazil
Meeting: 2013 American Transplant Congress
Abstract number: B931
Introduction: The early renal function benefit observed in belatacept (bela)-treated recipients of extended-criteria donor (ECD) kidneys in BENEFIT-EXT was maintained over time, with a consistent safety profile. Here we report changes in cGFR stage over 5 yrs in the long-term extension (LTE) cohort.
Methods: In BENEFIT-EXT, recipients of ECD kidneys were randomized to a more or less intensive (LI) regimen of bela or CsA. Pts remaining on assigned therapy after 3 yrs were eligible to enter the LTE. This posthoc analysis assessed shifts in cGFR stage between Months (Mos) 12 & 60 in the LTE cohort & focuses on the approved LI regimen. cGFR stages were defined per the KDOQI classification of CKD stages. cGFR was imputed as 0 for death or graft loss. Among pts enrolled in LTE, 103/113 LI & 79/87 CsA pts had cGFR data available at both Mos 12 & 60.
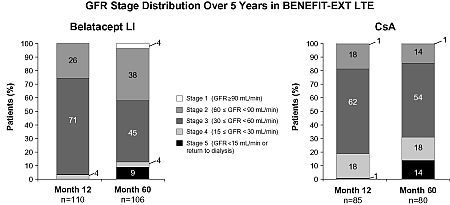
Results: GFR stage at Mos 12 & 60 in LI and CsA pts of the LTE cohort are shown in the Figure. No LI pts in the LTE cohort were in Stage 1 at Mo 12 & 1 CsA pt in Stage 1 at Mo 12 worsened to Stage 5 at Mo 60. Among pts in Stage 2 at Mo 12, 80% (20/25) LI & 47% (7/15) CsA pts maintained or improved their GFR stage through Mo 60. In Stage 3 pts, GFR stage from Mo 12–60 was maintained or improved in 85% (63/74) LI & 76% (38/50) CsA pts. In Stage 4 pts at Mo 12, 3/4 LI & 7/12 CsA pts maintained or improved their GFR stage through Mo 60. There were no LI pts in Stage 5 at Mo 12 in the LTE cohort, & 1/1 CsA pt improved from Stage 5 to Stage 4 from Mo 12–60.
Conclusions: In the BENEFIT-EXT LTE cohort, a smaller percentage of belatacept pts were in Stages 4 and 5 compared to CsA pts at 5 yrs. Belatacept pts receiving ECD kidneys were more likely than CsA pts to experience maintained or improved renal function over 5 yrs.
Durrbach, A.: Employee, BMS. Rial, M.: Grant/Research Support, Bristol-Myers Squibb. Lehner, F.: Other, BMS, Roche, Novartis, Honoraria, BMS, Roche, Novartis, Consulting Fee. Pupim, L.: Employee, BMS/Belatacept. Coffey, M.: Employee, Bristol-Myers Squibb. Pestana, J.: Grant/Research Support, Belatacept/BMS.
To cite this abstract in AMA style:
Grinyo J, Vanrenterghem Y, Durrbach A, Rial M, Charpentier B, Matas A, Lehner F, Pupim L, Coffey M, Pestana J. Likelihood of Improving or Maintaining Renal Function in Recipients of Extended-Criteria Donor Kidneys over Five Years with Belatacept or CsA (BENEFIT-EXT Long-Term Extension Study) [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/likelihood-of-improving-or-maintaining-renal-function-in-recipients-of-extended-criteria-donor-kidneys-over-five-years-with-belatacept-or-csa-benefit-ext-long-term-extension-study/. Accessed February 26, 2026.« Back to 2013 American Transplant Congress
