Twice Daily Telaprevir in Combination with Peginterferon Alfa-2a/Ribavirin in HCV Genotype 1 Liver Transplant Recipients: Interim Pharmacokinetics of the REFRESH Study
Mayo Clinic, Scottsdale, AZ
Vertex Pharmaceuticals Incorporated, Cambridge, MA
Henry Ford Hospital, Detroit, MI
Carolinas Medical Center, Charlotte, NC
University of British Columbia, Vancouver, BC, Canada
University of Michigan, Ann Arbor
Northwestern University, Chicago, IL
Columbia University, New York, NY
Meeting: 2013 American Transplant Congress
Abstract number: B1062
Background: REFRESH is a 2-part Phase 2b, open-label clinical study assessing safety, efficacy, and pharmacokinetics (PK) of telaprevir (T) in combination with peginterferon alfa-2a (P) and ribavirin (R) in noncirrhotic liver transplant patients (pts) with recurrent genotype 1 hepatitis C virus (HCV). Tacrolimus (TAC) and cyclosporine (CsA) are metabolized by CYP3A; coadministration with T, a CYP3A inhibitor, increases TAC and CsA exposure in healthy volunteers. In this interim analysis, PK of T, R, TAC or CsA when coadministered are reported in initial cohort (n=23).
Methods: Pts were on stable doses of TAC or CsA prior to therapy and received T 1125 mg BID + P 180 ug/wk + R 600 mg/day (initial dose) for 12 wks plus 36 wks of PR (no lead-in). Initial post-T TAC or CsA dose was reduced ∼10- (min. dose 0.5 mg) and ∼4-fold from baseline, respectively. Further dosing guided by TAC or CsA levels Days 1-5, 7, 10 and Wks 2, 3, 4, 6, 8, 10, 12 (investigator discretion). Intensive PK analysis for T performed Wk 4 (n=13). Random blood samples collected at Wk 4 for R.
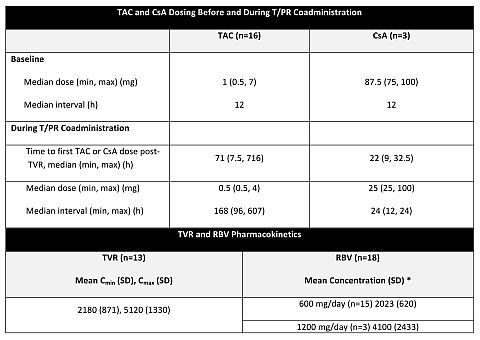
Results: Drug PK parameters are summarized in table. Three pts initiated R at 1200 mg/day, above protocol-specified dose; 2/3 had supratherapeutic R levels and developed Grade 3/4 anemia. R was reduced in 7 pts (including all 3 who started at 1200 mg) and increased in 3.
Conclusions: In initial subset of liver transplant pts, target levels of telaprevir were achieved (1125 mg BID). Target levels of R were achieved (initial dose 600 mg QD) with adjustments as necessary. When coadministered with telaprevir, TAC requires greater adjustment in dose and dosing interval than CsA.
Vargas, H.: Grant/Research Support, Vertex Pharmaceuticals Incorporated, Janssen/Tibotec, BMS, Novartis, Merck, Gilead. Dai, Y.: Employee, Vertex Pharmaceuticals Incorporated, Stockholder, Vertex Pharmaceuticals Incorporated. Brown, K.: Grant/Research Support, Exenenz, Speaker’s Bureau, Vertex Pharmaceuticals, Merck, Gilead, Other, Salix, Consultant, BCBS Transplant Centers, Consultant. Russo, M.: Grant/Research Support, Vertex Pharmaceuticals Incorporated, Speaker’s Bureau, Vertex Pharmaceuticals Incorporated. Yoshida, E.: Other, Vertex Pharmaceuticals Incorporated, CME Honouraria, Vertex Pharmaceuticals Incorporated, Clinical Trial Investigator, Janssen Inc. Clinical Trial Investigator, Vertex Pharmaceuticals Incorporated, Ad Board Honoraria. Fontana, R.: Grant/Research Support, Vertex Pharmaceuticals Incorporated, Gilead, Ocera, Other, Tibotec, Consultant, GSK, Consultant, Merck, Consultant. Levitsky, J.: Speaker’s Bureau, Vertex Pharmaceuticals Incorporated, Genentech. Rubin, R.: Employee, Vertex Pharmaceuticals Incorporated, Stockholder, Vertex Pharmaceuticals Incorporated. Garg, V.: Employee, Vertex Pharmaceuticals Incorporated, Stockholder, Vertex Pharmaceuticals Incorporated. Brown, R.: Other, Vertex Pharmaceuticals Incorporated, Consultant.
To cite this abstract in AMA style:
Vargas H, Dai Y, Brown K, Russo M, Yoshida E, Fontana R, Levitsky J, Rubin R, Garg V, Brown R. Twice Daily Telaprevir in Combination with Peginterferon Alfa-2a/Ribavirin in HCV Genotype 1 Liver Transplant Recipients: Interim Pharmacokinetics of the REFRESH Study [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/twice-daily-telaprevir-in-combination-with-peginterferon-alfa-2aribavirin-in-hcv-genotype-1-liver-transplant-recipients-interim-pharmacokinetics-of-the-refresh-study/. Accessed February 19, 2026.« Back to 2013 American Transplant Congress
