Pre-Transplant Angiotensin Receptor II Type 1 Antibodies (AT1R) and Serum Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) as Predictors of Post Transpalnt FSGS Recurrence
Indiana University school of Medicine-IU Health Transplant, Indianapolis, IN.
Meeting: 2015 American Transplant Congress
Abstract number: 358
Keywords: Recurrence, Renal dysfunction, Risk factors
Session Information
Session Name: Concurrent Session: Glomerulonephritis/Recurrent Disease
Session Type: Concurrent Session
Date: Tuesday, May 5, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:39pm-2:51pm
Presentation Time: 2:39pm-2:51pm
Location: Room 121-AB
Approximately 30% of renal transplant patients develop recurrence of FSGS in the first kidney allograft. AT1R is expressed on podocytes; its expression is elevated in the proteinuric state. This prompted us to test the pre-transplant sera of patients with history of biopsy proven FSGS for AT1R antibody levels along and serum soluble urokinase-type plasminogen activator receptor (suPAR) as a biomarker for risk of recurrence of FSGS after transplant.
Methods: Pre-transplant sera of 39 patients (FSGS 28, polycystic kidney disease [PKD] 11 as controls) were collected and stored at -80oC until testing in batch for anti-AT1R and suPAR under an IRB approved protocol. AT1R antibody and suPAR levels were measured using ELISA (One Lambda®, Canoga Park, CA) and suPAR ELISA kit.
Results: Twelve patients had biopsy proven post-transplant FSGS recurrence at 1.5 months. No difference was found in the pre-transplant suPAR levels of FSGS patients (5993±2292pg./mL)vs.PKD (7334±4538pg./mL),(p= 0.23)..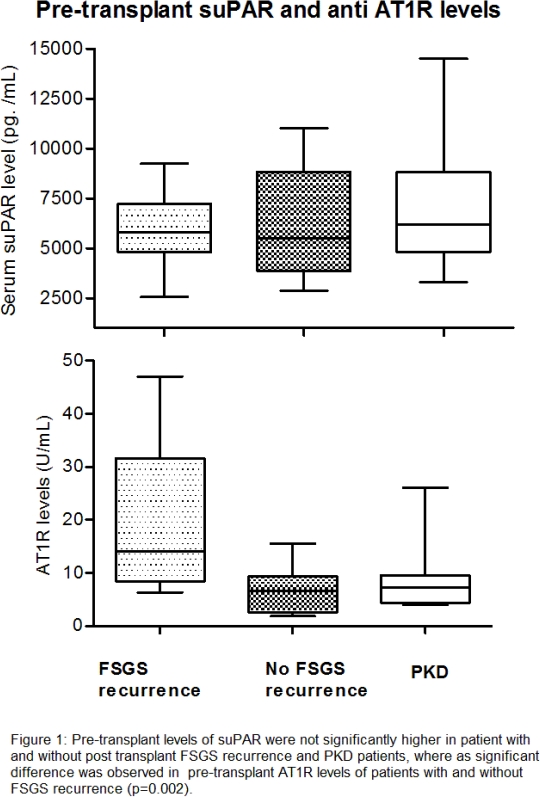 Serum suPAR levels in patients with FSGS recurrence (5786±1899pg./mL) vs. no FSGS recurrence(6149±2598pg./mL)(p= 0.69) were not different.. Mean AT1R antibody levels in patients with FSGS were 12.66±11.85 U/mL vs. 8.69±6.52 U/mL in PKD (p= 0.32), however, a difference was found in patients with 20.41±14.36 U/ml and without FSGS recurrence 6.84±4.181 U/mL (p<0.01). Area under curve for suPAR and AT1R to predict post-transplant FSGS recurrence was 0.51 and 0.84 respectively.
Serum suPAR levels in patients with FSGS recurrence (5786±1899pg./mL) vs. no FSGS recurrence(6149±2598pg./mL)(p= 0.69) were not different.. Mean AT1R antibody levels in patients with FSGS were 12.66±11.85 U/mL vs. 8.69±6.52 U/mL in PKD (p= 0.32), however, a difference was found in patients with 20.41±14.36 U/ml and without FSGS recurrence 6.84±4.181 U/mL (p<0.01). Area under curve for suPAR and AT1R to predict post-transplant FSGS recurrence was 0.51 and 0.84 respectively. 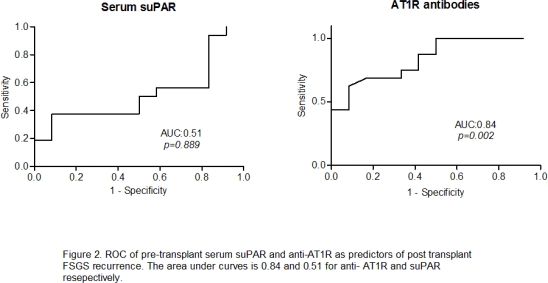
Conclusion: Pre-transplant anti-AT1R antibody levels appear to be a helpful biomarker in identifying patients at high risk of post-transplant FSGS recurrence. More studies are needed to confirm our findings.
To cite this abstract in AMA style:
Khalil A, Tim T, Sharfuddin A, Doshi S, Yaqub M, Mishler D, Goggins W, Mujtaba M. Pre-Transplant Angiotensin Receptor II Type 1 Antibodies (AT1R) and Serum Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) as Predictors of Post Transpalnt FSGS Recurrence [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/pre-transplant-angiotensin-receptor-ii-type-1-antibodies-at1r-and-serum-soluble-urokinase-type-plasminogen-activator-receptor-supar-as-predictors-of-post-transpalnt-fsgs-recurrence/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
