Evaluation of a Change in Pharmacy Discharge Medication Education for Kidney Transplant Recipients
1Pharmacy, Nebraska Medicine, Omaha, NE, 2College of Pharmacy, University of Nebraska Medical Center, Omaha, NE
Meeting: 2022 American Transplant Congress
Abstract number: 1666
Keywords: Kidney, Kidney transplantation, Outcome, Patient education
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: To evaluate if modification of pharmacy discharge education for kidney transplant recipients contributed to a decreased length of hospital stay (LOS) post-transplant.
*Methods: This was a single center, retrospective cohort study that included all adult kidney ± pancreas transplant recipients from an academic hospital from July 1, 2017 to June 30, 2020. Patients were excluded if they received a multiorgan transplant or a second transplant during the index period. Patients were placed into three cohorts based on where pharmacy teaching was completed: Group 1 (7/1/2017- 6/30/2018) – inpatient; Group 2 (7/1/2018- 6/30/2019) – inpatient+outpatient follow-up; Group 3 (7/1/2019- 6/30/2020) – focused immunosuppression inpatient teaching+comprehensive outpatient follow-up, which included a filled pillbox on discharge and pharmacist follow-up visit within 1 week. The primary endpoint was LOS. Secondary endpoints included readmission within 30 days, cause of readmission, biopsy-proven acute cellular rejection (BPAR) and antibody mediated rejection (AMR) within 6 months post-transplant, time from discharge orders placed to patient exiting the hospital, and patient and graft survival at 6 months post-transplant. Analysis included descriptive statistics, ANOVA, and adjustments with ANCOVA.
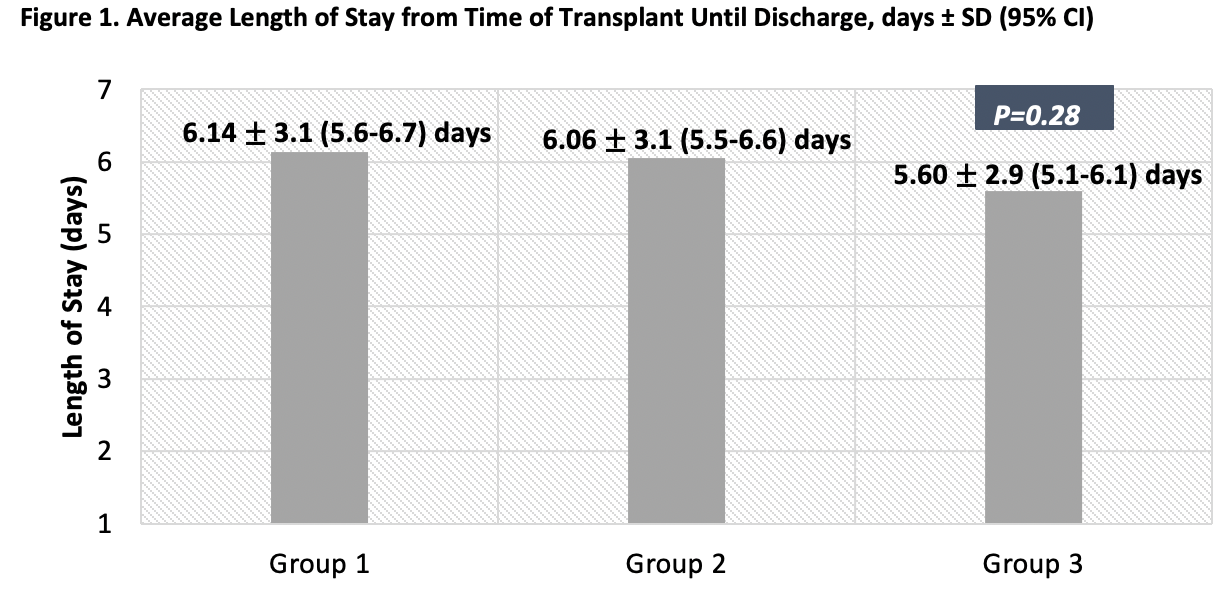
*Results: The study included 412 patients; 130 in Group 1, 139 Group 2, and 143 in Group 3. Demographics were similar between groups. Average LOS (days) was numerically longer, but not statistically different between Groups 1, 2 and 3, respectively (6.14 ± 3.1 [5.6-6.7] vs 6.06 ± 3.1 [5.5-6.6] vs 5.60 ± 2.9 [5.1-6.1], p =0.28). In Groups 1, 2, and 3 respectively, the time of discharge orders placed to discharge in hours remained approximately unchanged (7.0 ±7.3 vs 6.8 ± 6.9 vs 6.1 ± 3.6, p = 0.38), 30-day readmission rates were similar (28.5% vs 32.4% vs 34.3%, p=0.166) and medication reasons for readmission were minimal for all groups (<1% of readmissions). Other secondary outcomes were also similar between groups: BPAR (3.8% vs 2.2% vs 7.7%, p=0.079), AMR (5.4% vs 2.2% vs 0.7%, p=0.06), patient survival (100% vs 99.3% vs 100%, p=0.73), and graft survival (99.2% vs 98.6% vs 97.9% p=0.879). Adjusted results were similar to the primary findings.
*Conclusions: Adjusting pillbox education to outpatient follow-up has not adversely affected outcomes of kidney transplant recipients. The observed trend towards shorter LOS warrants further investigation in a larger cohort and controlling for severity of illness.
To cite this abstract in AMA style:
Borsh M, Leick M, Donovan A, McAdam-Marx C, Keck M. Evaluation of a Change in Pharmacy Discharge Medication Education for Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-a-change-in-pharmacy-discharge-medication-education-for-kidney-transplant-recipients/. Accessed January 20, 2026.« Back to 2022 American Transplant Congress