Outcomes in Renal Transplant Recipients Receiving Pre-Transplant Financial Assistance Through the American Kidney Fund
1Department of Pharmacy, University of Colorado Hospital, Aurora, CO, 2Department of Surgery, University of Colorado Hospital, Aurora, CO, 3Department of Renal Med Diseases/Hypertension, University of Colorado Hospital, Aurora, CO
Meeting: 2022 American Transplant Congress
Abstract number: 1735
Keywords: Kidney transplantation, Medicare, Rejection, Risk factors
Topic: Clinical Science » Kidney » 50 - Health Equity and Access
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
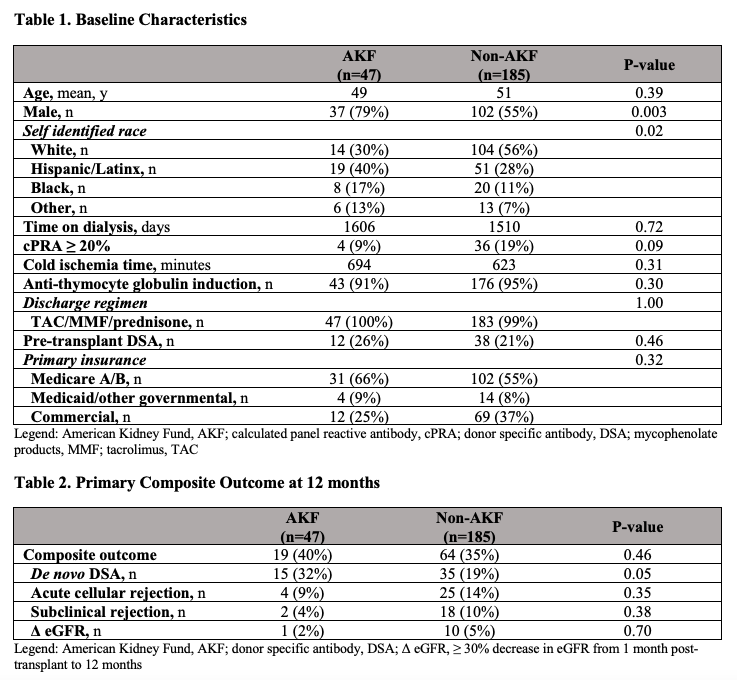
*Purpose: Pre-transplant financial assistance for insurance premiums is available for eligible patients through the American Kidney Fund (AKF), but this funding ends within the first year of transplant. The purpose of this study is to evaluate post-transplant outcomes in patients who receive financial assistance through AKF at the time of transplant compared to those who do not and to describe the relationship between AKF funding and transplant outcomes.
*Methods: The present study, comparing patients with AKF funding versus those without, was a single center, retrospective cohort that included 232 adult patients who received a kidney alone transplant with patient survival > 30 days post transplant. A composite of de novo donor specific antibody (dnDSA) formation, acute cellular rejection, subclinical rejection, or eGFR decrease ≥ 30% from baseline 1 month after transplant to 12 months was utilized as the primary outcome. Categorical outcomes were evaluated utilizing Chi-squared or Fisher’s exact test while continuous data was analyzed utilizing a t-test. Analysis was completed using RStudio® (Version 1.2.5019). The institutional review board approved this project.
*Results: Baseline demographics were similar between groups, except for gender (Table 1). The proportion of patients with the primary composite outcome at 12 months (Table 2) was 64/185 (35%) in the non-AKF cohort and 19/47 (40%) in the AKF cohort (p=0.46). For components of the composite outcome, differences in subclinical rejection, acute cellular rejection, and eGFR decrease were nonsignificant (Table 2). However, the proportion of patients with dnDSA was higher in the AKF cohort (32%) compared to the non-AKF cohort (19%), although this difference was not statistically significant (p=0.053).
*Conclusions: A significant difference in the primary composite outcome (dnDSA, acute cellular rejection, subclinical rejection, or eGFR decrease) at 12 months was not detected. However, a greater percentage of AKF patients developed dnDSA. This data highlights the need for additional research to characterize the impact of such funding on post-transplant outcomes. Further study may elucidate the patients at increased risk post-transplant, allowing development of strategies to mitigate risk and decrease disparities. This data will be further expanded upon and 24 month outcomes will be evaluated.
To cite this abstract in AMA style:
Roppo H, Klem P, Crowther B, Sartain E, Schoeppler K, Kennealey PT, Kaplan B. Outcomes in Renal Transplant Recipients Receiving Pre-Transplant Financial Assistance Through the American Kidney Fund [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-in-renal-transplant-recipients-receiving-pre-transplant-financial-assistance-through-the-american-kidney-fund/. Accessed February 9, 2026.« Back to 2022 American Transplant Congress