Management and Outcomes of Coronavirus Disease 2019 in Solid Organ Transplant Recipients
Baylor St. Luke's Medical Center, Houston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1659
Keywords: COVID-19, Immunosuppression, Infection
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The purpose of this study was to assess treatment of coronavirus disease 2019 (COVID-19) and immunosuppression modification in solid organ transplant (SOT) recipients, and identify risk factors that contribute to mortality among SOT recipients with COVID-19.
*Methods: The primary endpoint was 90-day mortality. Secondary endpoints include COVID-19 treatment regimen, laboratory abnormalities, maintenance immunosuppression modification, concomitant infections, complications such as renal replacement therapy (RRT) and more. This retrospective chart review included all SOT recipients who presented to our institution with a positive COVID-19 test over an 18-month period. Dual organ transplant recipients were excluded.
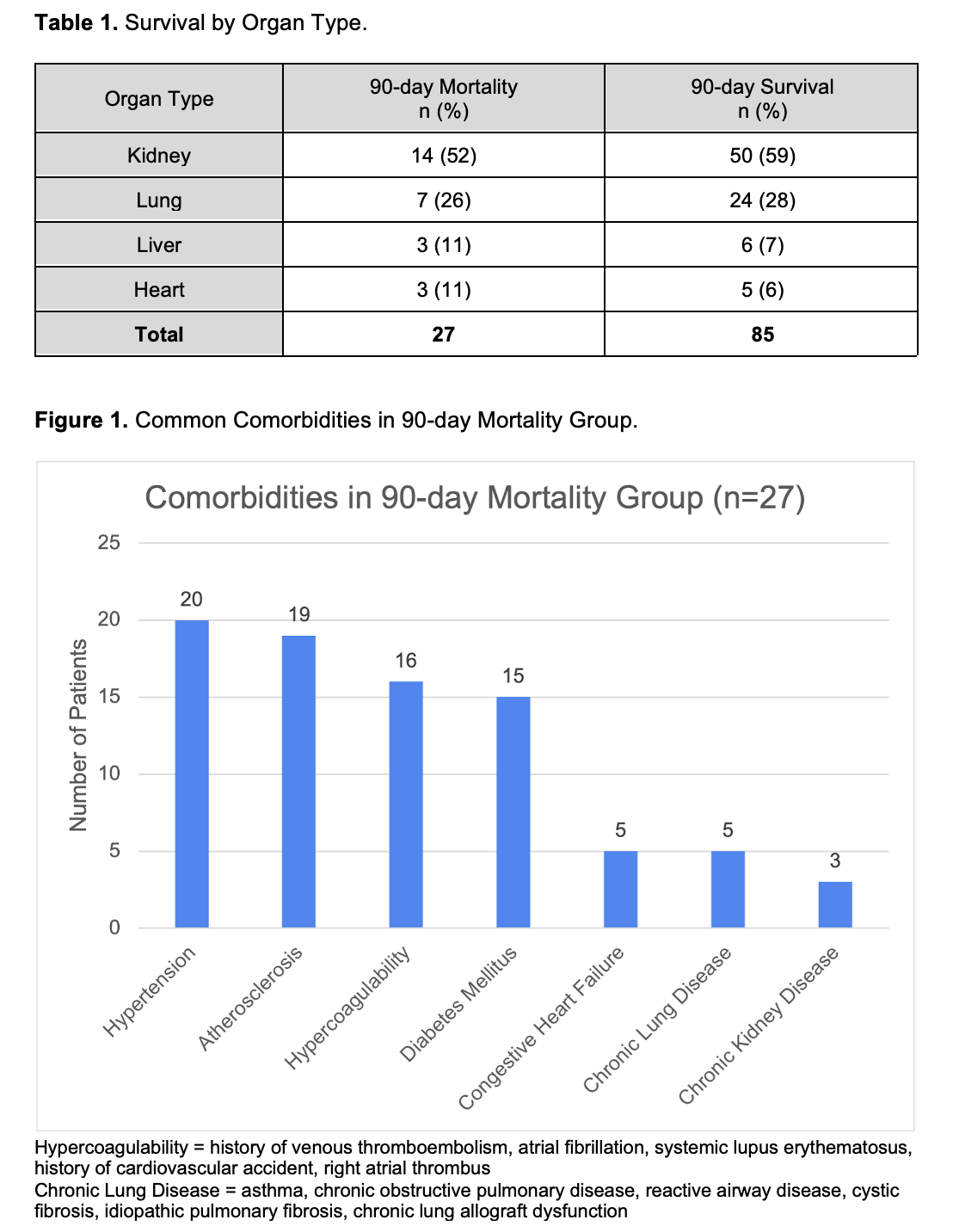
*Results: A total of 112 patients met inclusion criteria. The average age was 56 ± 13 years and 58% of patients were male. The majority of patients identified as Latino (39%), followed by African-American (29%) and Caucasian (28%). Forty-nine percent required treatment in the intensive care unit. Inflammatory markers were elevated in the majority of patients. Most immunosuppression modification strategies held antimetabolites and prednisone if receiving treatment with glucocorticoids. The primary endpoint of 90-day mortality was observed in 24% (27/112) of patients. Mortality rates by organ type can be found in Table 1. Common comorbidities in these patients are depicted in Figure 1. There was no significant difference in mortality between patients receiving remdesivir 12/27 vs. 20/85 (p=0.05). A 50% (4/8) mortality rate was observed among patients who received rabbit antithymocyte globulin within 6 months of COVID-19 infection (p=0.09). Concomitant respiratory infections showed a statistically significant difference in mortality 12/27 vs. 9/85 (p<0.05). All 6 patients that required extracorporeal membrane oxygenation expired. Fifty-two percent (14/27) of patients in the mortality group required RRT vs. 7% (6/85) of those that survived (p<0.05). While most cases were prior to the advent of COVID-19 vaccines, all 9 patients that received at least one dose of any COVID-19 vaccine prior to infection survived at 90 days.
*Conclusions: Concomitant respiratory infections and RRT were significant predictors of mortality among SOT recipients with COVID-19. Administration of rabbit antithymocyte globulin within 6 months of COVID-19 infection and treatment with remdesivir should be investigated in future studies as they may demonstrate significant associations in a larger sample size.
To cite this abstract in AMA style:
Scott A, Truman Z, Manson M. Management and Outcomes of Coronavirus Disease 2019 in Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/management-and-outcomes-of-coronavirus-disease-2019-in-solid-organ-transplant-recipients/. Accessed February 4, 2026.« Back to 2022 American Transplant Congress