Organ Transplant Outcomes from Donors with Positive SARS-CoV-2 PCRs in New York: A Case Series
A. L. Friedman1, M. Pereira2, M. Rana3, J. Akpede4, A. Dhand5
1LiveOnNY, New York, NY, 2New York Presbyterian Hospital, New York, NY, 3Icahn School of Medicine at Mount Sinai, New York, NY, 4LiveOnNY, Long Island City, NY, 5Westchester Medical Center, Chappaqua, NY
Meeting: 2022 American Transplant Congress
Abstract number: 245
Keywords: COVID-19, Donation, Infection, Procurement
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: COVID-19 Infections Part 2: All Organs
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:50pm-4:00pm
Presentation Time: 3:50pm-4:00pm
Location: Hynes Ballroom B
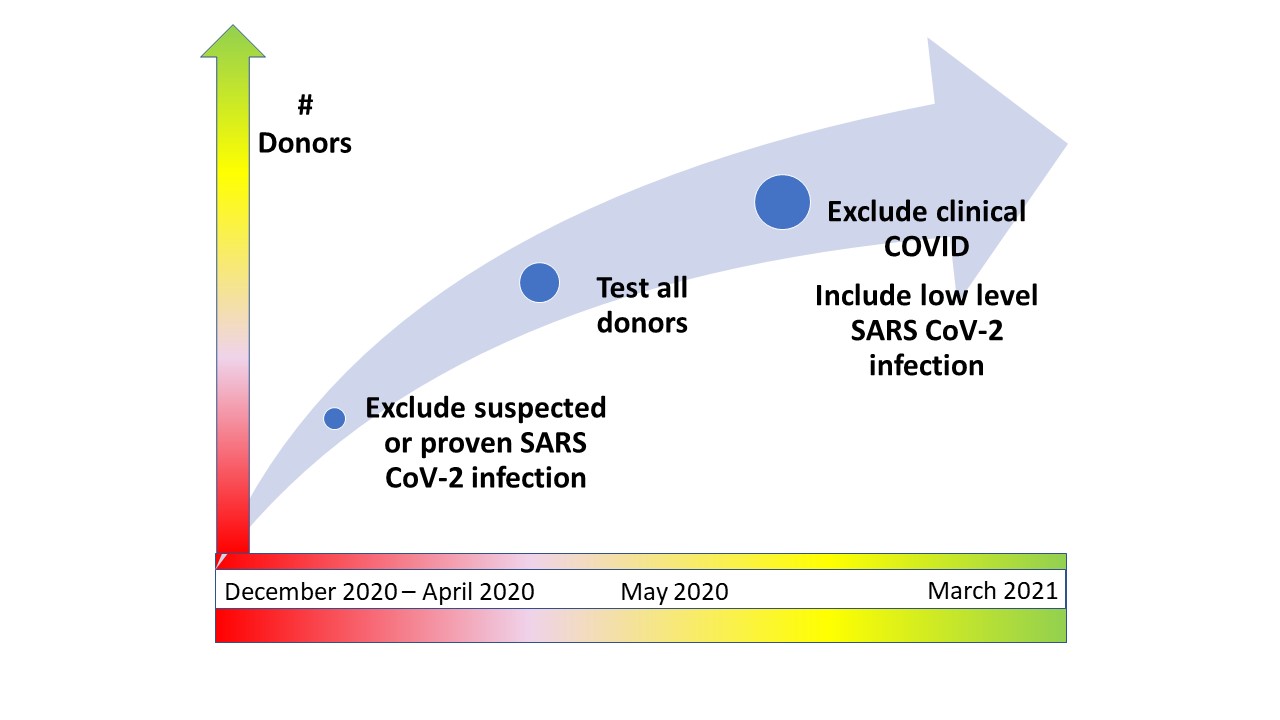
*Purpose: Rapid evolution of the SARS-CoV-2 pandemic over the past 24 months has demanded agility in managing selection criteria for deceased organ donors, with the goal of saving every possible life while avoiding disease transmission to recipients. At 1 large organ procurement organization (OPO), the detection of any SARS-CoV-2 in a naso-pharyngeal (NP) specimen by polymerase chain reaction (PCR) was initially an absolute contraindication to organ donation. That approach gradually became more refined utilizing clinical evidence along with detection of low levels of viremia.
*Methods: A retrospective analysis of all patients with authorization for organ donation after brain death or circulatory death from 3/16/2020 – 11/9/2021 was undertaken. Patients with any positive result for a COVID-19 test were identified. Donors with any positive result of an NP +/- broncho-alveolar fluid (BAL) PCR were selected for this analysis. Organ allocation was accompanied by the expectation of written confirmation that the recipient had provided informed consent for use of an organ from a SARS-CoV-2 donor.
*Results: A total of 18 deceased donors from whom 49 organs were transplanted, were identified. Multiple test results were often available for a single patient. Results were mixed in all 18 donors. At least one of the positive NP PCR test results included a cycle threshold in 16/18 patients and ranged from 31.4 to 42.5. In 2 donors a BAL PCR was also positive; 1 heart was donated from one of these donors. With a follow-up of > 53 days for all transplants, no known transmission of SARS-CoV-2 to recipients or transplant teams has been reported.
*Conclusions: Available laboratory testing for SARS-CoV-2 and deepening understanding of COVID-19, increasing treatment options, and evolution of infection prevention practices have facilitated a growing confidence in safely transplanting non-lung organs from donors with a positive SARS-CoV-2 test.
| Organs Transplanted | Kidney | Liver | Heart | Pancreas | Lung |
| 49 | 28 | 12 | 6 | 1 | 2 |
To cite this abstract in AMA style:
Friedman AL, Pereira M, Rana M, Akpede J, Dhand A. Organ Transplant Outcomes from Donors with Positive SARS-CoV-2 PCRs in New York: A Case Series [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/organ-transplant-outcomes-from-donors-with-positive-sars-cov-2-pcrs-in-new-york-a-case-series/. Accessed February 18, 2026.« Back to 2022 American Transplant Congress