Utility of Protocol Pancreas Biopsies for De Novo Donor-Specific Antibodies
University of Wisconsin, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 1181
Topic: Clinical Science » Pancreas » 65 - Pancreas and Islet: All Topics
Session Information
Session Name: Pancreas and Islet: All Topics
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Donor-specific antibodies (DSA) against the human leukocyte antigens (HLA) can now be measured accurately and repetitively, and are routinely monitored post-transplant. In recipients of various other solid organ transplants, this monitoring improves outcomes through early diagnosis of subclinical rejection. Here, we evaluate the utility of DSA monitoring and protocol biopsies in response to denovo DSA (dnDSA) in pancreas transplant recipients (PTRs) with stable pancreatic enzymes.
*Methods: At our center, we recently protocolized routine monitoring of post-transplant DSA in all PTRs, followed by protocol biopsy after the detection of dnDSA.
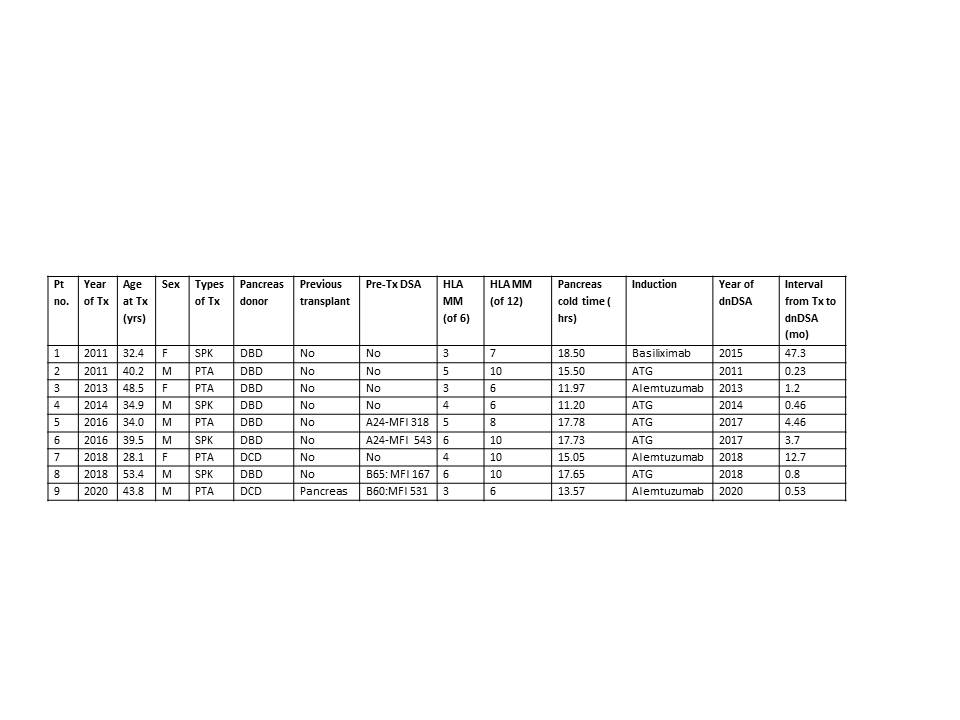
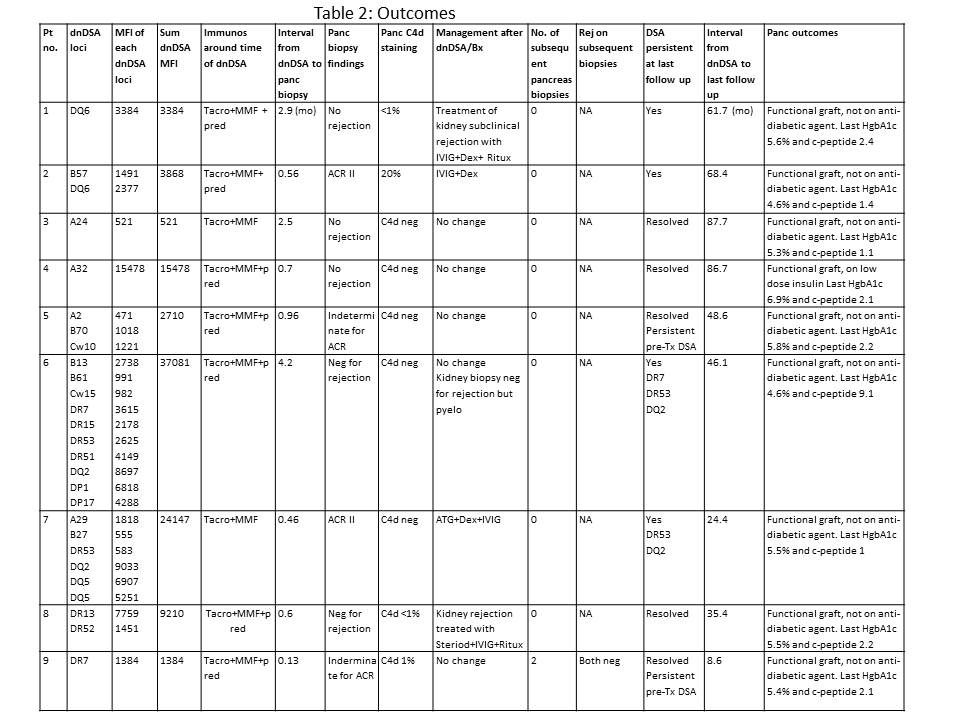
*Results: A total of 9 PTRs, 4 simultaneous pancreas and kidney (SPK), and 5 pancreas transplants alone (PTA), underwent protocol pancreas biopsy for dnDSA, all in the presence of normal pancreatic enzymes. The basic demographics of these PTRS are presented in Table 1. Of these, 2 PTRS, both PTA, had subclinical TCMR, and 2 additional PTA had indeterminate pancreas rejection (Table 2). 3 PTRS had dnDSA against class I antigen only, 3 against class II antigen only, and 3 had a mixture of both class I and II. The most common dnDSA specificities were against DQ and DR, each in 4 PTRs. Both PTA recipients with subclinical rejection had functional grafts at last follow up, which was more than 2 and 5 years post-biopsy, respectively. Among the 4 SPK recipients, none had pancreas rejection; however, 2 had subclinical kidney AMR.
*Conclusions: Although limited by small sample size, our data support the utility of serial DSA monitoring followed by protocol biopsy for dnDSA and stable graft function among PTRs, similar to other solid organ transplants.
To cite this abstract in AMA style:
Parajuli S, Mandelbrot D, Odorico J. Utility of Protocol Pancreas Biopsies for De Novo Donor-Specific Antibodies [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/utility-of-protocol-pancreas-biopsies-for-de-novo-donor-specific-antibodies/. Accessed February 9, 2026.« Back to 2022 American Transplant Congress