Case-Control Study of Belatacept as Maintenance Immunosuppression in Kidney Transplant Recipients Living with HIV
M. Tucker, C. Foppiano Palacios, E. Cohen, S. Virmani, P. Trubin, L. A-Barakat, M. M. Azar, M. Malinis
Yale School of Medicine, New Haven, CT
Meeting: 2022 American Transplant Congress
Abstract number: 988
Keywords: HIV virus, Immunosuppression, Infection, Kidney transplantation
Topic: Clinical Science » Infection Disease » 25 - Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Information
Session Name: Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Belatacept has been increasingly used in kidney transplant recipients (KTR) as a calcineurin-inhibitor (CNI) sparing agent. There are little data on belatacept use in KTR living with HIV (KTRLH). This study is designed to evaluate graft and infection outcomes of KTRLH transitioned to belatacept.
*Methods: We conducted a retrospective case-control study of KTRLH transitioned to belatacept at Yale from March 2014 – September 2020. KTRLH who received belatacept (Group A) were matched by age at transplant in a 1:2:2 ratio to a cohort of KTRLH who did not receive belatacept (Group B) and a cohort of KTR without HIV who received belatacept (Group C). Medical records were reviewed for demographics, graft and patient survival, and infections. Descriptive analysis was performed.
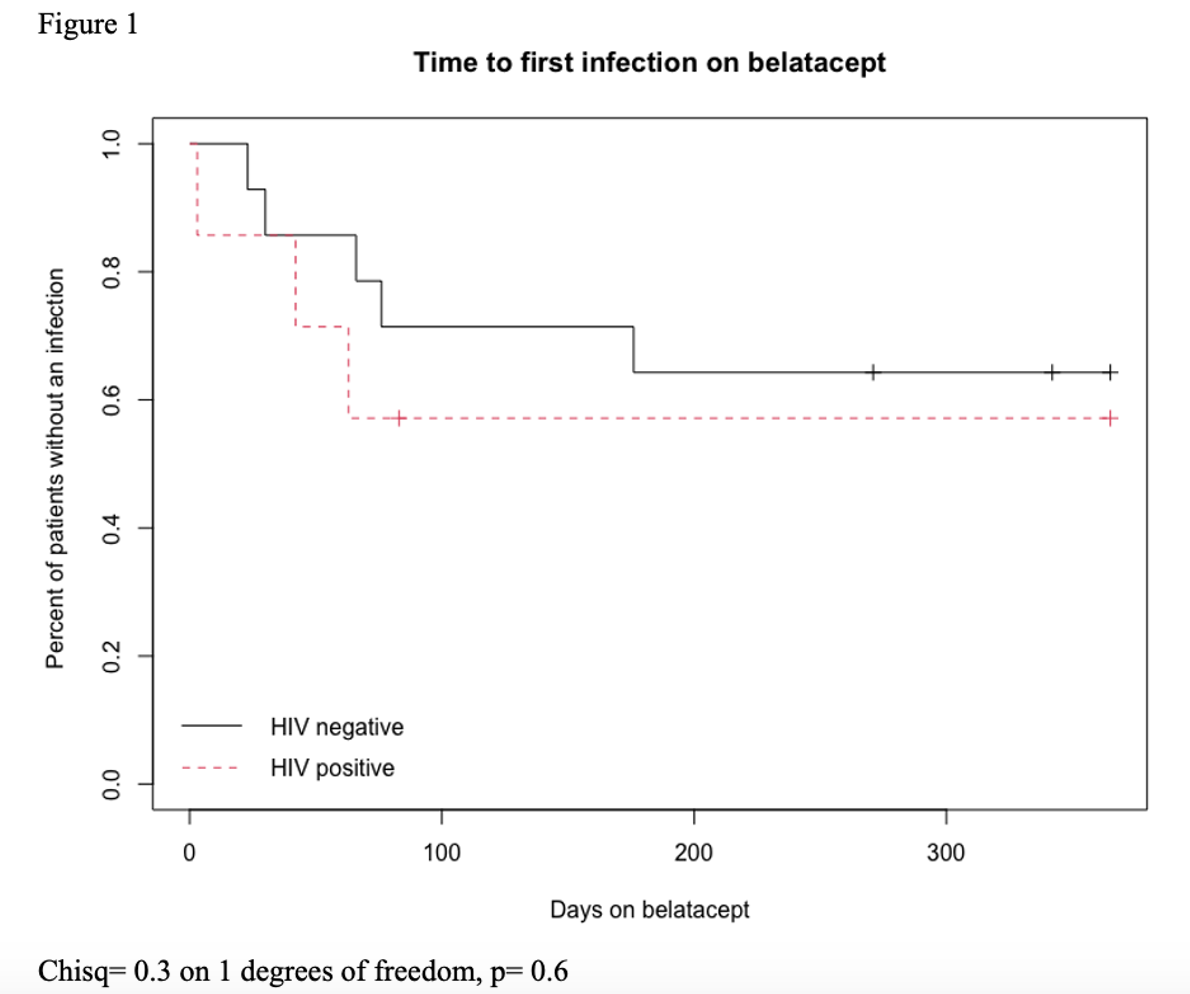
*Results: Thirty-five KTR were included (Group A n=7, Group B n=14, Group C n=14). The majority of KTR in Groups A and B were black, male and had a higher Charlson comorbidity index compared to Group C (Table 1). Within a year of belatacept initiation, 3/7 patients (43%) in Group A developed infections (bacterial pneumonia, influenza, Enterococcus bacteremia) vs. 5/14 (36%) in Group C (influenza, UTI, CMV viremia, PJP, nocardiosis). Time to first infection within one year of belatacept conversion was similar between Groups A and C (Figure 1). All three groups had similar graft survival (86%) by last follow-up (Table 1). However, patient survival was higher in Group C (93%) compared to Groups A and B (71%).
*Conclusions: In this study, there were no apparent differences in graft outcomes or infection rates in KTR on belatacept regardless of HIV status. Patient survival was decreased for KTRLH regardless of belatacept use. Though limited by small cohort, there was no evident disadvantage to the use of belatacept in KTRLH. Future multi-center studies are needed to further evaluate belatacept use in KTRLH.
To cite this abstract in AMA style:
Tucker M, Palacios CFoppiano, Cohen E, Virmani S, Trubin P, A-Barakat L, Azar MM, Malinis M. Case-Control Study of Belatacept as Maintenance Immunosuppression in Kidney Transplant Recipients Living with HIV [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/case-control-study-of-belatacept-as-maintenance-immunosuppression-in-kidney-transplant-recipients-living-with-hiv/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress