Covid-19 in Pediatric Kidney Transplant: A Follow-Up Report of the Improving Renal Outcomes Collaborative
C. Varnell, L. Harshman, M. Seifert, D. Hooper
Cincinnati Children's Hospital Medical Center, Cincinnati, OH
Meeting: 2022 American Transplant Congress
Abstract number: 164
Keywords: COVID-19, Kidney transplantation, Pediatric
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: COVID-19 Infections Part 1: All Organs
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:30pm-6:40pm
Presentation Time: 6:30pm-6:40pm
Location: Hynes Ballroom B
*Purpose: To date studies of children with a kidney transplant and COVID-19 are limited. The Improving Renal Outcomes Collaborative (IROC) is a learning health network in the US comprised of 36 pediatric kidney transplant programs that provides infrastructure to efficiently conduct multicenter studies.
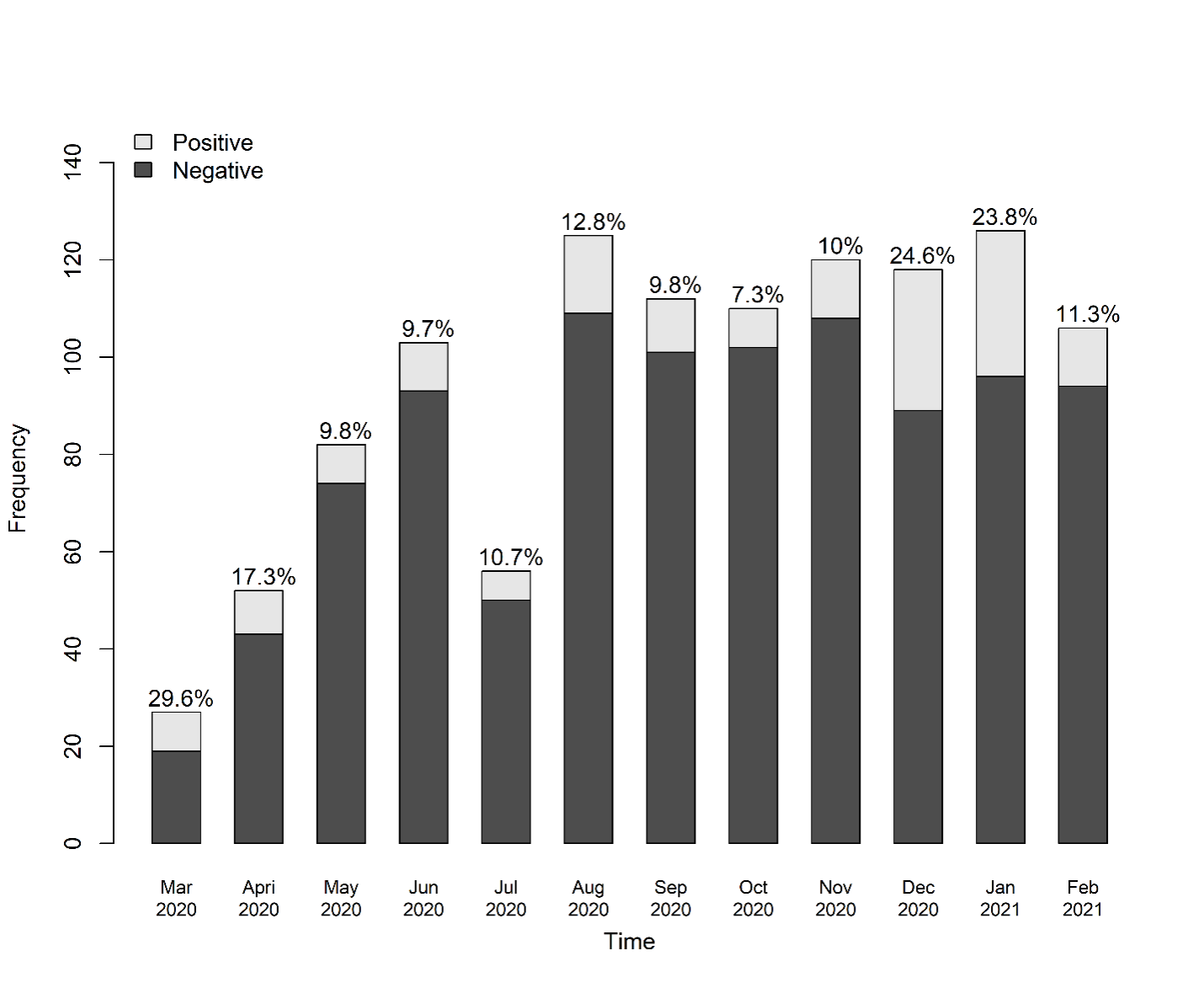
*Methods: We collected COVID-19 testing, indications, and outcomes data. Data were linked to patient demographic and clinical data in the IROC registry. We previously reported the results of this effort from April 6-September 3, 2020 (era 1). Here we report data from September 4, 2020 – February 28, 2021 (era 2). We describe the differences in testing frequency and positive testing over the two eras.
*Results: In era 1, 22 centers submitted testing data; in era 2, 21 centers submitted testing data. There were 281 tests in 281 patients analyzed in era 1 and 648 tests for 465 patients in era 2. From era 1 to era 2, the proportion of positive tests increased from 24/281 (8.54%) to 109/465 (23%). Testing frequency and results from eras 1 and 2 are displayed in Figure 1. 133 patients tested positive for COVID-19 over both eras, and there was no difference in the symptoms at the time of testing between the two eras. The most common symptoms for both eras were fever (35%), cough (33%), rhinorrhea (23%), vomiting (14%), and diarrhea (14%). Over both eras, 41/133 (31%) that tested positive for COVID-19 had no symptoms at the time of testing. There were no differences in outcomes between the two eras. 117 patients (88%) had no transplant complications. 1 (0.8%) patient had T cell-mediated rejection, 3 (2%) had antibody-mediated rejection, 1 (0.8%) had mixed T cell- and antibody-mediated rejection, 10 (7.5%) had acute kidney injury, 1 (0.8%) experienced graft failure, and 1 patient (0.8%) died from their COVID-19 infection.
*Conclusions: In this cohort, there was overall more testing and more positive COVID-19 tests in era 2. The dates of era 2 correspond with children returning to school and the Winter surge in cases in the US. Despite the increased number and proportion of positive patients, the clinical outcomes are consistent with reported outcomes for their non-immunosuppressed peers. Follow-up studies will be required to evaluate whether the availability of vaccines for adolescents and the rise of the delta variant as the predominant strain affect clinical outcomes
To cite this abstract in AMA style:
Varnell C, Harshman L, Seifert M, Hooper D. Covid-19 in Pediatric Kidney Transplant: A Follow-Up Report of the Improving Renal Outcomes Collaborative [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/covid-19-in-pediatric-kidney-transplant-a-follow-up-report-of-the-improving-renal-outcomes-collaborative/. Accessed January 29, 2026.« Back to 2022 American Transplant Congress