Covid-19 Vaccination Response In Tacrolimus Treated Kidney Transplant Recipients With And Without Mycophenolate Mofetil: Follow-up Of A Randomized Controlled Trial
1Department of Internal Medicine, Erasmus MC Transplant Institute University Medical Center, Rotterdam, Netherlands, 2Department of Internal Medicine, Groningen Medical Center, Groningen, Netherlands
Meeting: 2022 American Transplant Congress
Abstract number: 9008
Keywords: COVID-19, Immunosuppression, Kidney transplantation, Mycophenolate mofetil
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Late Breaking: COVID-19
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 2:00pm-3:00pm
 Presentation Time: 2:10pm-2:20pm
Presentation Time: 2:10pm-2:20pm
Location: Hynes Room 310
*Purpose: To investigate the effect of mycophenolate mofetil (MMF) on SARS-CoV-2 vaccination response in kidney transplant recipients using the standard immunosuppressive regimen of tacrolimus (TAC) and MMF.
*Methods: A randomized controlled trial in immunologically low risk kidney transplant recipients
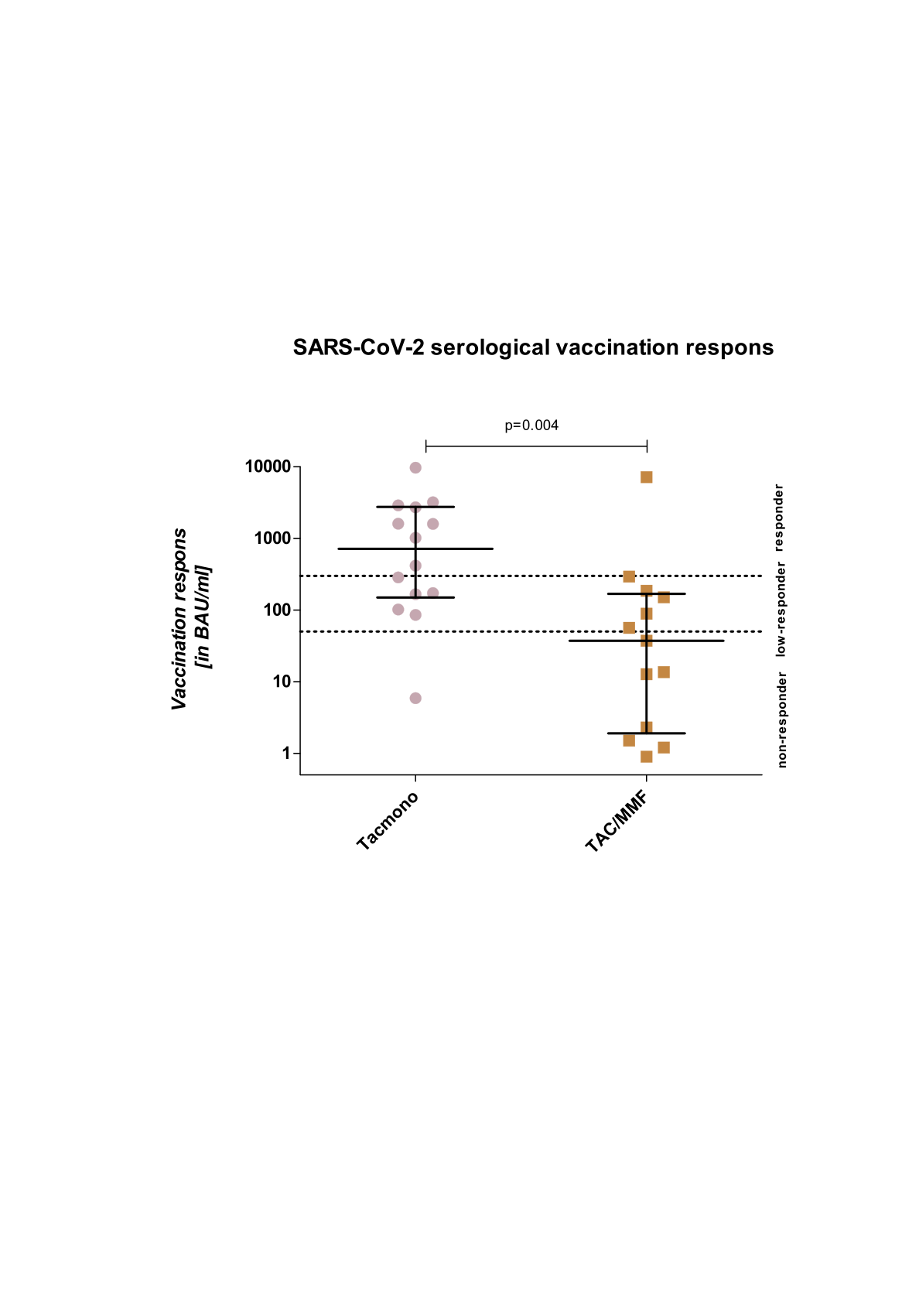
was performed (EudraCT nr.: 2014-001372-66). Patients were randomized to standard TAC/MMF or TAC monotherapy (TACmono) from 9 months onwards, without steroids. Antibody based immune responses to SARS-CoV-2 vaccination (mRNA-1273 or BNT162b2) were investigated in a central laboratory, as part of the RECOVAC Antibody study (EudraCT nr.: 2021-283 001520-18), 4-8 weeks after the second vaccination. Measurement involved the presence of antibodies against the receptor binding domain (RBD) of the SARS-CoV-2 S-protein (IgG anti-RBD antibody) using the Sanquin anti-SARS-CoV-2 RBD IgG ELISA assay. Patients were classified as non-responders (≤50 BAU/mL), low-responders (50-300 BAU/mL) and responders (>300 BAU/mL).
*Results: Between 2015 and 2018, 79 recipients were randomized to TAC/MMF (n=41) and TACmono (n=38). At the outbreak of the COVID-19 pandemic in early 2020, 67 patients were alive with a functioning graft (TAC/MMF n=35, TACmono n=32). In 27 patients antibody responses could be established: Ten patients were excluded from the analyses due to symptomatic COVID-19 infection and 1 due to a positive nucleocapsid test, possibly from an asymptomatic infection. The rest did not participate in the vaccination study, because of ChAdOx1-S, age >80 years or lack of informed consent. Mean age was 64 (43-75) years, median time after transplantation 4.2 (3.0-6.5) years and eGFR was 53 (36-105) ml/min/1.73m2. TAC trough levels were 6.6 (±0.3) µg/L in both groups, and MMF dose was 1000 mg daily (range 500-2000) in TAC/MMF. Median SARS-CoV-2 Spike S1-specific IgG antibody levels were 37.3 BAU/ml in TAC/MMF (5 non, 7 low, 1 responder) and 715.6 BAU/ml in TACmono (1 non, 6 low, 7 responders, p =0.004, figure 1). Of note is that antibody levels of >1000 BAU/ml, as a presumed threshold for protection against Omicron (B.1.1.529), was reached in 1/13 TAC/MMF and 7/14 TACmono patients (p=0.03).
*Conclusions: In this controlled study mycophenolate mofetil on top of tacrolimus severely hampered serological COVID-19 vaccination response.
To cite this abstract in AMA style:
Fatly ZAl, Betjes MG, Messchendorp AL, Sanders JS, Reinders ME, Kho MM, Weerd AEde. Covid-19 Vaccination Response In Tacrolimus Treated Kidney Transplant Recipients With And Without Mycophenolate Mofetil: Follow-up Of A Randomized Controlled Trial [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/covid-19-vaccination-response-in-tacrolimus-treated-kidney-transplant-recipients-with-and-without-mycophenolate-mofetil-follow-up-of-a-randomized-controlled-trial/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress