Cardiovascular Outcomes of Kidney Transplant Recipients Receiving Belatacept Compared to a Matched Tacrolimus
Y. Kim1, J. Marvin1, K. Belfield1, G. Girone1, R. Formica2, E. Cohen1
1Pharmacy, Yale New Haven Hospital, New Haven, CT, 2Section of Nephrology, Yale School of Medicine, New Haven, CT
Meeting: 2022 American Transplant Congress
Abstract number: 787
Keywords: Hyperglycemia
Topic: Clinical Science » Kidney » 35 - Kidney: Cardiovascular and Metabolic Complications
Session Information
Session Name: Kidney: Cardiovascular and Metabolic Complications
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Tacrolimus (TAC), a calcineurin inhibitor (CNI), is the backbone of maintenance immunosuppression in kidney transplant recipients (KTR) but has been associated with cardiovascular (CV) events. As CV disease is the leading cause of death in KTR, various CNI-free regimens have been explored. Belatacept (BELA), a co-stimulatory inhibitor, is an alternative with a favorable adverse event profile; however, there is limited data comparing its CV effects with TAC. This study was designed to investigate CV effects of BELA compared to TAC in KTR.
*Methods: In this retrospective cohort study, KTR who received BELA either de novo or conversion from 1/2013-10/2020 were matched 1:1 to TAC based on transplant date, age, duration of dialysis, body mass index (BMI), pre-transplant diabetes and any history of cardiovascular accident (CVA), myocardial infarction (MI), coronary artery bypass graft (CABG), percutaneous coronary intervention (PCI) or peripheral artery disease revascularization. Patients with less than 3 months of follow-up or 1 follow-up annually were excluded. The primary outcome was the composite of hospitalization for heart failure, CVA, MI, CABG, PCI and CV death. Secondary outcomes included graft failure, acute rejection, death, post-transplant diabetes (PTDM) and change in BMI and hemoglobin A1c (HbA1c). Outcomes were assessed starting from the date of initial BELA dose or equivalent time post-transplant for the TAC cohort and were analyzed using Fisher’s exact test, chi-square test, Wilcoxon test, Student’s t-test, two-way ANOVA and repeated-measures mixed-effects models where appropriate.
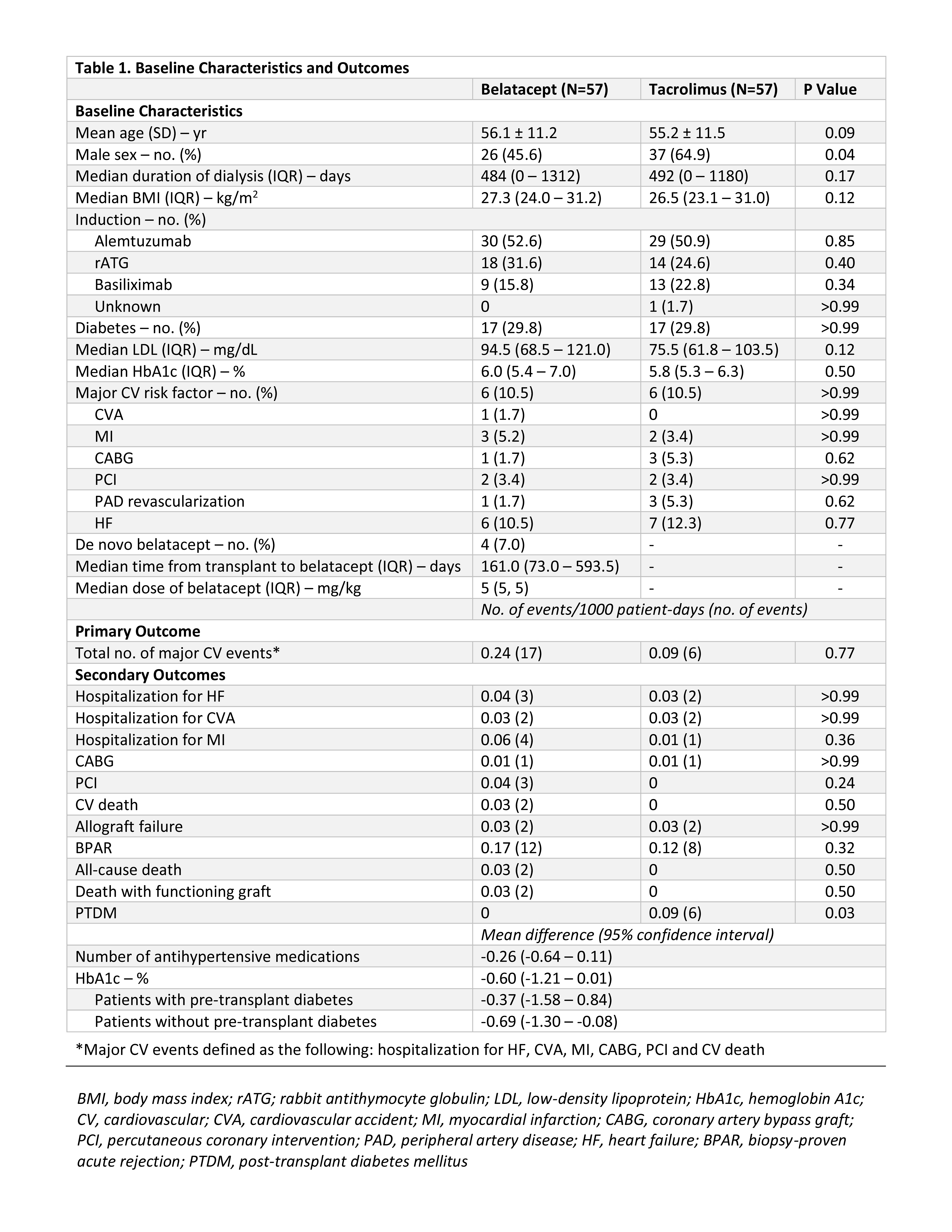
*Results: A total of 114 KTR were included in the analysis. Mean duration of follow-up (±SD) was 1267 ± 639 days on BELA and 1210 ± 625 days on TAC. There was no significant difference in baseline characteristics or the primary outcome (P=0.77) (Table 1). PTDM was observed less frequently with BELA compared to TAC (P=0.03). HbA1c decreased significantly with BELA compared to TAC in KTR without preexisting diabetes (Mean Difference, -0.69; 95% CI, -1.30 to -0.08).
*Conclusions: BELA did not reduce major CV events compared to TAC. BELA was associated with decreased incidence of PTDM overall and a reduction in HbA1c in patients without pre-transplant diabetes. Further studies are needed to assess long term CV outcomes.
To cite this abstract in AMA style:
Kim Y, Marvin J, Belfield K, Girone G, Formica R, Cohen E. Cardiovascular Outcomes of Kidney Transplant Recipients Receiving Belatacept Compared to a Matched Tacrolimus [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/cardiovascular-outcomes-of-kidney-transplant-recipients-receiving-belatacept-compared-to-a-matched-tacrolimus/. Accessed December 27, 2025.« Back to 2022 American Transplant Congress