The Development of a Scientific Registry of Transplant Recipients Data Query Tool: A Human-Centered Design Process
1Hennepin Healthcare Research Institute, Minneapolis, MN, 2Hennepin Healthcare (HHS) / University of Minnesota (UMN), Minneapolis, MN, 3Accenture Federal Services, Washington, DC
Meeting: 2022 American Transplant Congress
Abstract number: 606
Keywords: Monitoring, Outcome, Patient education
Topic: Administrative » Administrative » 01 - Quality Assurance Process Improvement & Regulatory Issues
Session Information
Session Name: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
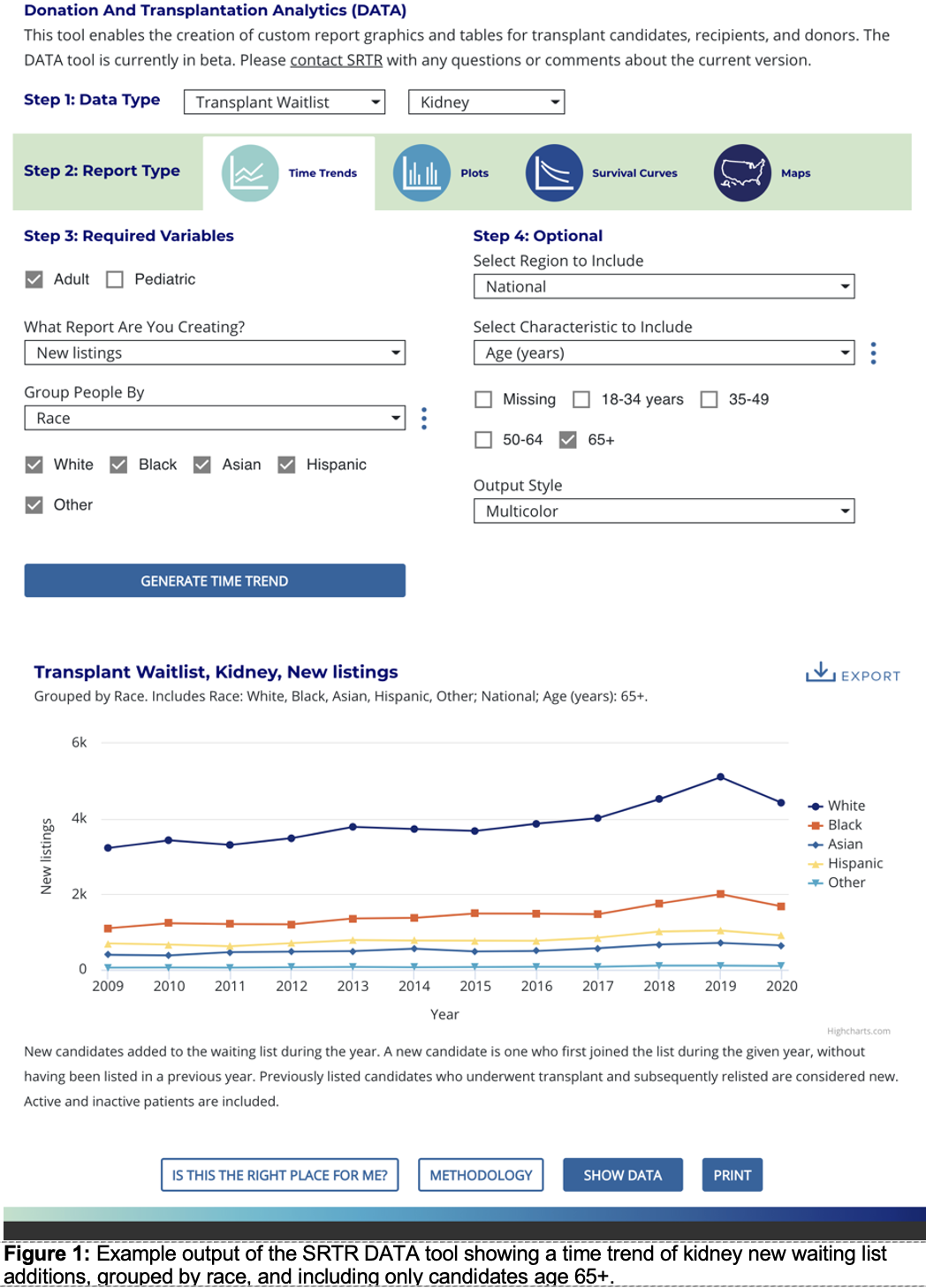
*Purpose: The Scientific Registry of Transplant Recipients (SRTR) provides national transplant data in various forms, including the OPTN/SRTR Annual Data Report (ADR). The ADR includes numerous static plots and tables; however, customization is not possible in print form. To increase the usefulness and accessibility of national transplant data to stakeholders, SRTR developed a new interactive online data query tool to allow customization and multiple graphical output styles. We sought to use a human-centered design process to engage stakeholders and iteratively improve the design.
*Methods: The development included multiple steps: 1) Developing maps of the data structure of candidate, recipient, and donor data; 2) Benchmarking review of existing external data tools; 3) Development of multiple user interface concepts; 4) Conducting virtual focus groups of existing SRTR data users, including professional, patient, and government stakeholders; 5) Refining concepts based on feedback and internal review with a committee of design professionals; 6) Developing database infrastructure to support desired user interface features.
*Results: The duration of the development process was about 9 months. Four focus groups included a convenience sample of 23 participants [patients (2), researcher (4), quality improvement / staff (5), clinician (2), organ procurement organization (5), industry (1), and government (4)]. The iterative concept development included a series of 8 rounds of mockups. Mockups presented multiple graphical output types: time trends, plots, survival curves, and maps. Final designs included preferred features based on internal and focus-group reviews.
*Conclusions: The final tool, named the Donation and Transplantation Analytics (DATA) tool, is available in beta form on the SRTR public website. Our design process can serve as a template for future human-centered design of transplant decision support tools, metrics, and reports for system monitoring. Future work will review ongoing feedback of the DATA tool to continue iterative improvements using the human-centered design process.
To cite this abstract in AMA style:
Schaffhausen C, Hart A, Zinner CS, Israni AK, Snyder J. The Development of a Scientific Registry of Transplant Recipients Data Query Tool: A Human-Centered Design Process [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/the-development-of-a-scientific-registry-of-transplant-recipients-data-query-tool-a-human-centered-design-process/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress