Use of Donor-Derived Cell-Free DNA in Children with Kidney Transplantation: A Pilot Study
UCSF, San Francisco, CA
Meeting: 2021 American Transplant Congress
Abstract number: 1023
Topic: Clinical Science » Kidney » Kidney: Pediatrics
Session Information
Session Name: Kidney: Pediatrics
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Use of a blood test to detect donor-derived cell-free DNA (dd-cfDNA) levels has been shown to predict rejection in adults with kidney transplants. If dd-cfDNA levels perform similarly in children, use of this biomarker for monitoring pediatric recipients could lead to earlier detection of rejection and potentially reduce the need for biopsy. We aim to determine whether dd-cfDNA is a useful marker of rejection in children by designing a cohort study with serial assessments of dd-cfDNA levels after kidney transplantation. The primary objective of this phase of the study was to assess feasibility of participant recruitment and ascertainment of dd-cfDNA levels as well as parent/patient perspectives on its potential application in routine care.
*Methods: This is a single-center prospective study of children treated with kidney transplant. At enrollment, participants (if >8 years) or their parents (if /=2 months post-transplant with stable creatinine. Baseline dd-cfDNA levels were compared between children <13 vs. >/=13 years using Wilcoxon Rank-Sum test.
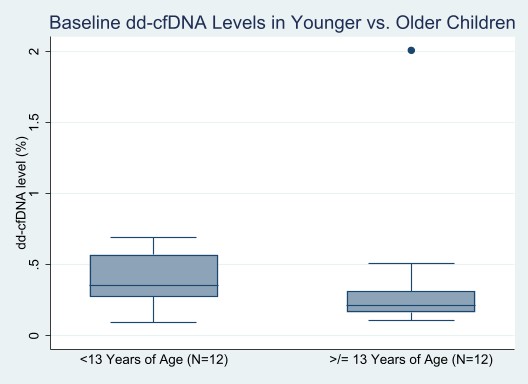
*Results: Of 29 patients approached for participation, 27 (93%) consented to enroll. Median (Q1, Q3) age at entry was 13 (5, 16) years, 56% were male, 37% were non-Hispanic white race, and the primary cause of ESKD was congenital anomalies of the kidney and urinary tract (48%). Participants or their parents (for younger children) were least concerned about blood draws, with a median (Q1,Q3) rating of 1 (1,1). Median (Q1, Q3) rating for level of concern of undergoing kidney biopsy was 3 (1,5). Of 25 participants with stable creatinine, baseline dd-cfDNA levels were ascertained in 24 (96%) at a median (Q1,Q3) of 23.6 (5.0, 69.0) months post-transplant; one sample was hemolyzed. Median (Q1,Q3) baseline dd-cfDNA level was 0.29% (0.19,0.48) in the overall cohort. Levels were not significantly different for younger versus older children (median 0.36% vs. 0.21%, p=0.11, Figure).
*Conclusions: Recruitment of participants and ascertainment of dd-cfDNA levels is feasible in this prospective cohort study. Baseline dd-cfDNA are similar in younger children compared to adolescents to date. This ongoing study will follow dd-cfDNA levels and aims to determine whether dd-cfDNA levels will discriminate risk of rejection.
To cite this abstract in AMA style:
Winnicki E, Brennan J, McEnhill M, Brakeman P, Ku E. Use of Donor-Derived Cell-Free DNA in Children with Kidney Transplantation: A Pilot Study [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/use-of-donor-derived-cell-free-dna-in-children-with-kidney-transplantation-a-pilot-study/. Accessed February 16, 2026.« Back to 2021 American Transplant Congress