Belatacept Conversion in Elderly Renal Transplant Recipients
1UW Health, Madison, WI, 2University of Wisconsin School of Medicine and Public Health, Madison, WI
Meeting: 2021 American Transplant Congress
Abstract number: 931
Keywords: Co-stimulation, Elderly patients, Immunosuppression, Kidney transplantation
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Describe the impact of conversion from a calcineurin inhibitor (CNI) based immunosuppressive regimen to belatacept in elderly renal transplant recipients.
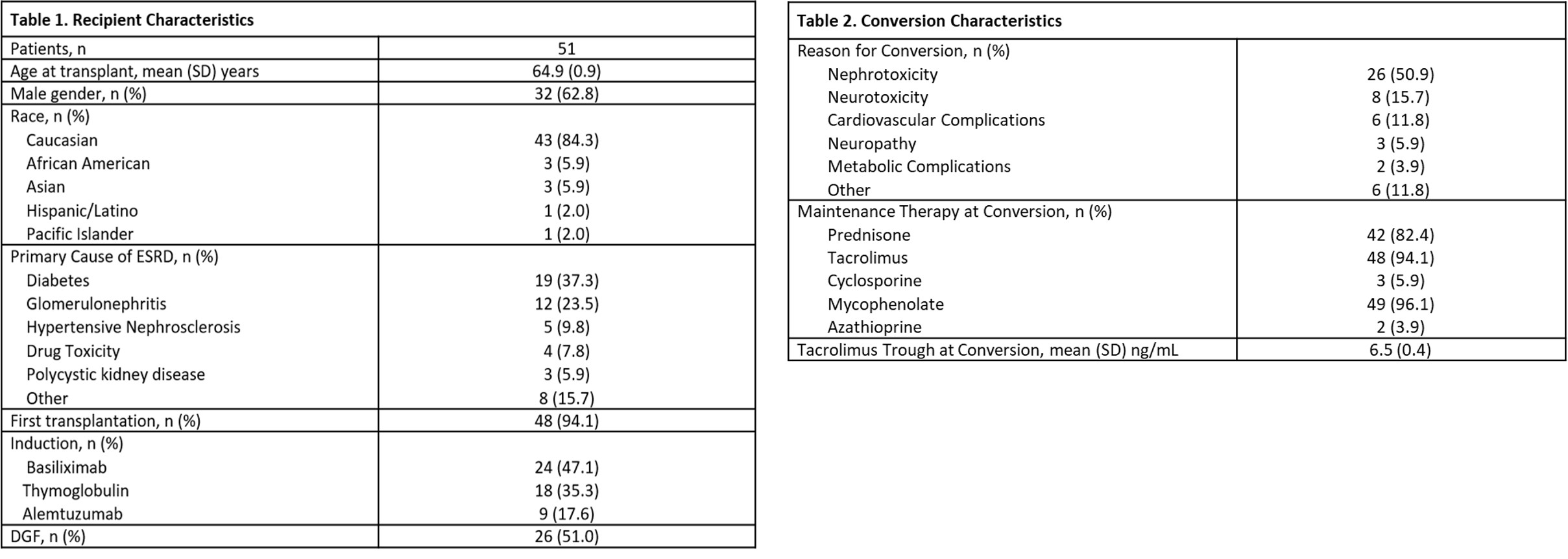
*Methods: Adult renal transplant patients aged ≥ 60 years who were converted to belatacept from a CNI between 3/2013-6/2020 were included. Belatacept conversion was defined as CNI withdrawal or minimization (cyclosporine trough < 75 ng/mL or tacrolimus < 5 ng/mL) following belatacept initiation. Primary objective was to describe our experience with the safety of conversion defined as 6 month incidence of infection and malignancy. Secondary endpoints included efficacy defined as change in estimated GFR (eGFR), incidence of rejection, death-censored graft loss and death at last follow-up.
*Results: Fifty-one patients met inclusion criteria, 86% with CNI withdrawal and 14% with minimization. Median time to conversion was 6.3 (IQR 2.3-20) months post-transplant with median follow-up of 12 (IQR 7-27) months. Mean age at time of conversion was 67 (SD 0.8) years. Conversion was pursued due to desire to improve renal function in 51%, neurotoxicity in 22% and metabolic/cardiovascular concerns in 16% of patients. Discontinuation of belatacept occurred in 27% of patients during follow-up. Primary reason for discontinuation was rejection (29%) followed by infection (21%). There were no instances of transplant lymphoproliferative disorder. However, 7.8% of patients experienced other malignancies. Thirty-nine percent of patients experienced at least one infection within 6 months of conversion with mean time from conversion to first infection of 1.8 (SD 0.4) months. Bacterial infections occurred in 27%, viral in 24%, and fungal in 2% of patients. eGFR significantly improved following belatacept conversion (31 ml/min/1.73m2 pre vs 42 ml/min/1.73m2 post; p = 0.002). Rejection occurred in 20% of patients at a median of 2 (IQR 1.9-2.7) months post conversion. There was a cumulative incidence of 5.7 death-censored graft losses per 100 person-years and 10.3 deaths per 100 person-years.
*Conclusions: In our observational study, conversion from a CNI based regimen to belatacept in elderly renal transplant recipients resulted in improved eGFR. However, infection occurred in over a third of the population, which is at higher risk of infection due to age-related immune senescence, and rejection in a fifth of patients. The discontinuation rate was over 25%. Further studies evaluating safety, particularly infectious risk, in elderly patients converted to belatacept are warranted.
To cite this abstract in AMA style:
Durst M, Felix D, Jorgenson M, Descourouez J, Astor BC, Mandelbrot D. Belatacept Conversion in Elderly Renal Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-conversion-in-elderly-renal-transplant-recipients/. Accessed January 11, 2026.« Back to 2021 American Transplant Congress