Successful Single-Center Experience Using HCV+ Organs for Single and Dual-Organ Transplantation
S. Aslam, C. Logan, N. Law, J. Kerr, J. Kozuch, V. Ajmera, K. Afshar, A. Khan, E. Adler, K. Mekeel, E. Golts, V. Pretorius
UCSD, San Diego, CA
Meeting: 2021 American Transplant Congress
Abstract number: 810
Keywords: Hepatitis C
Topic: Clinical Science » Infectious Disease » Non-Organ Specific: Viral Hepatitis
Session Information
Session Name: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: National data demonstrate that increasing organ transplantation opportunities exist from hepatitis C virus (HCV)-infected individuals to HCV negative recipients.
*Methods: We developed a clinical practice protocol in 2017 for the acceptance of HCV+ donors for HCV-negative patients who underwent solid organ transplantation. HCV+ donors included both viremic, i.e. HCV nucleic acid test positive (NAT+) as well as non-viremic, i.e. HCV antibody positive but NAT negative (Ab+/NAT-). Recipients were treated with direct acting antivirals (DAA) after documentation of infection transmission. After obtaining institutional review board approval, we retrospectively reviewed the outcomes at our institution.
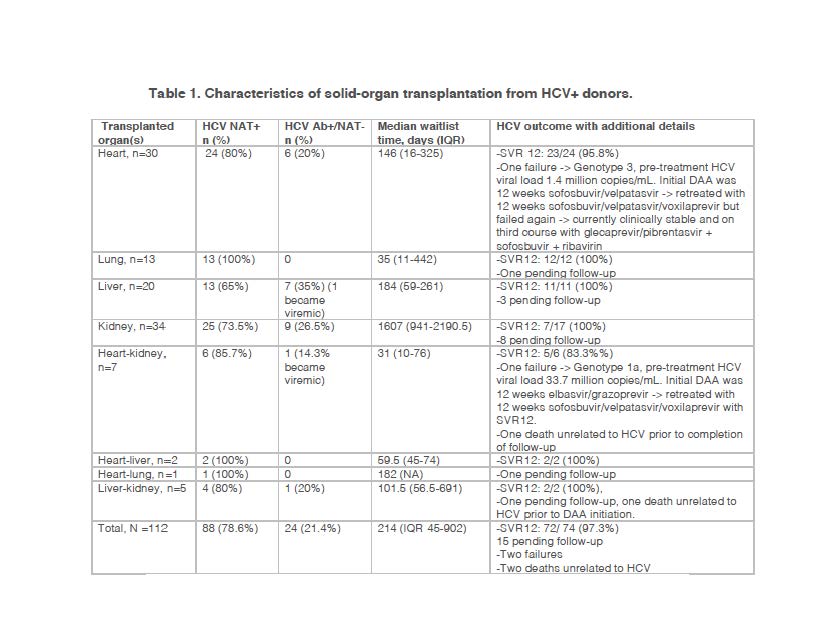
*Results: We transplanted 112 patients from HCV+ donors during the time-period 4/1/2017-10/31/2020 (Table 1). 97 patients received a single organ transplant and 15 received dual organ transplant. Median age of recipients was 59 years (IQR 49.5-65.5), 83/112 (75.5%) were male and median waitlist time was 214 days (IQR 45-902). All 88 patients receiving HCV NAT+ organs developed HCV viremia and 87 were treated in the post-operative period with 12 weeks of DAA. One recipient died soon after transplant from non-HCV related reasons and was not initiated on DAA. Additionally, 2/24 (16.7%) patients receiving HCV Ab+/NAT- organs developed HCV viremia (liver 1, heart-kidney 1) and were also treated with 12 weeks of DAA. DAA used included glecaprevir/pibrentasvir (68), sofosbuvir/velpatasvir (15), elbasvir/grazoprevir (2) and ledipasvir/sofosbuvir (4) with drug choice determined by patient’s medical insurance coverage. Among patients treated with DAA, sustained virological response at 12 weeks after end of DAA therapy (SVR12), indicative of a treatment cure, was achieved in 72/74 (97.3%) recipients. 15 DAA- treated recipients are pending follow-up to assess for SVR12, two recipients failed the initial DAA course, one died prior to DAA initiation of unrelated cause and one died after completion of DAA but prior to completion of 12 weeks of follow-up to determine SVR12.
*Conclusions: We report successful single-center experience using HCV+ organs for both single and dual solid organ transplant. As experience using such organs for transplant continues to increase and long term follow-up is obtained, HCV+ donors may be routinely used to increase the organ donor pool.
To cite this abstract in AMA style:
Aslam S, Logan C, Law N, Kerr J, Kozuch J, Ajmera V, Afshar K, Khan A, Adler E, Mekeel K, Golts E, Pretorius V. Successful Single-Center Experience Using HCV+ Organs for Single and Dual-Organ Transplantation [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/successful-single-center-experience-using-hcv-organs-for-single-and-dual-organ-transplantation/. Accessed January 14, 2026.« Back to 2021 American Transplant Congress