Comparison of Outcomes in Sot Recipients During Two Eras of Covid-19 Therapeutics
T. Chiang, A. Sait, A. Massie, K. Marr, D. Brennan, W. Cochran, P. Shah, T. Jain, S. Mehta Steinke, N. Desai, N. Permpalung, S. Shoham, C. Merlo, C. Durand, W. Werbel, M. Dioverti, D. Ostrander, A. Gurakar, M. Y. Kim, J. Garonzik-Wang, D. Segev, R. Avery
Johns Hopkins University, Baltimore, MD
Meeting: 2021 American Transplant Congress
Abstract number: 726
Keywords: Renal function
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: COVID-19 therapies have evolved over time, but little is known regarding outcomes in SOT recipients treated with newer therapeutic agents such as remdesivir, dexamethasone, and convalescent plasma. We sought to compare outcomes including mortality, rejection, and renal function in a retrospective cohort of SOT recipients with COVID-19 treated during two different eras of therapy.
*Methods: 40 SOT recipients hospitalized for COVID-19 at our center comprised Era 1 (Mar- May 2020, 20 patients) and Era 2 (Jun – Aug 2020, 20 patients). Data were collected on demographics, comorbidities, renal function, and mortality at time points out to 90 days after COVID-19 infection.
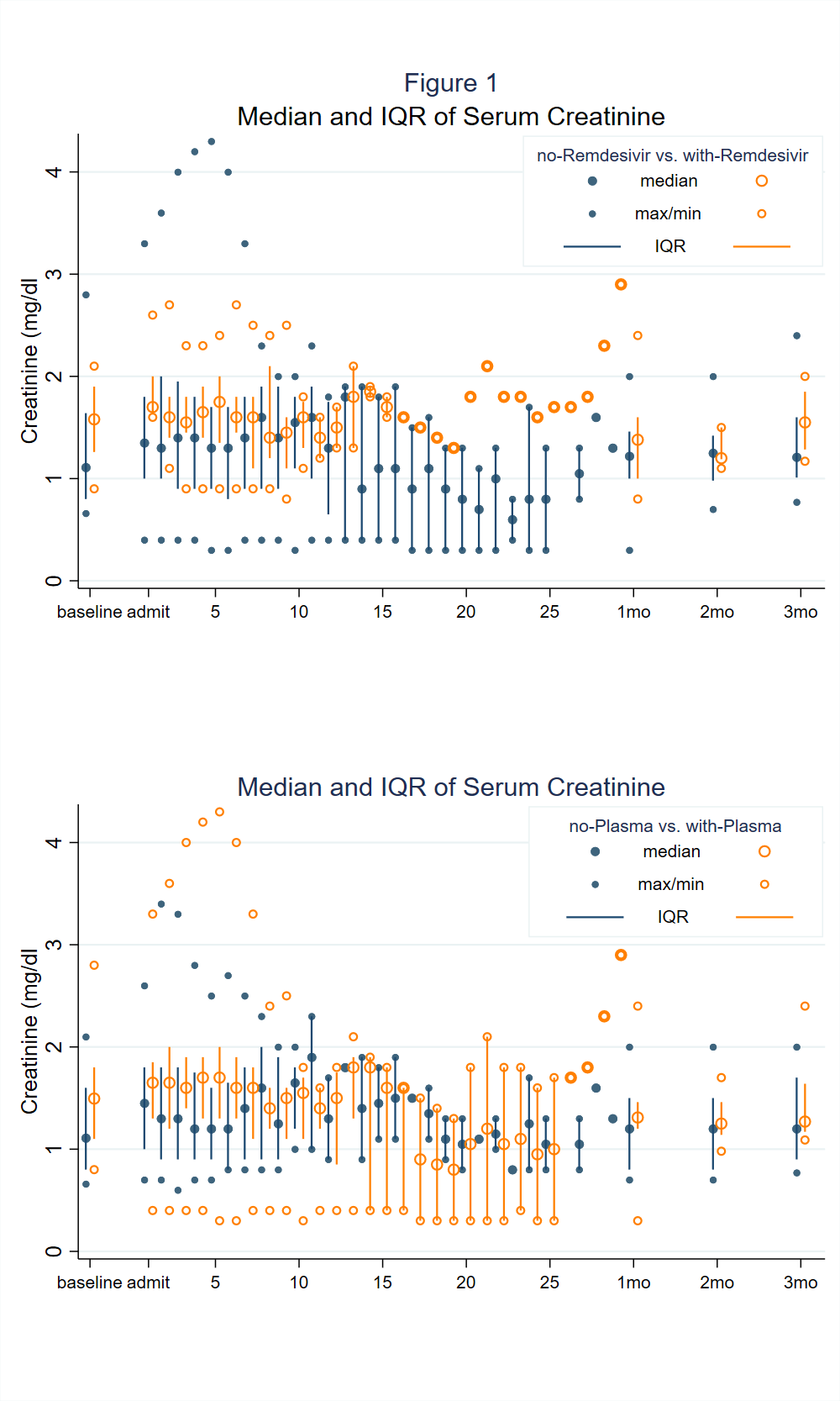
*Results: Patients in Era 1 received hydroxychloroquine (11/20, 55%), tocilizumab (5/20, 25%) and/or convalescent plasma (3/20, 15%) as targeted therapy; patients in Era 2 received primarily remdesivir (8/20, 40%), dexamethasone (6/20, 30%), and/or convalescent plasma (13/20, 65%). Mortality was 1/20 in Era 1 and 0/20 in Era 2. MMF was held in 33/35 (94%) of patients. Acute kidney injury was present on presentation in 14/40 (35%). The median (IQR) decrease in SCr (mg/dl) between admission and last followup was 0.5 (0.4-0.6) and 0.1 (0-0.4) in patients who had and had not received remdesivir, respectively (p=0.02), 0.5 (0.1-0.6) and 0.1 (0-0.3) in patients who had and had not received plasma, respectively (p=0.09). Antibody-mediated rejection (AMR) occurred in 2 patients in Era 1 and 0 patients in Era 2. Acute cellular rejection (ACR) occurred in 1 patient in Era 1 and 0 patients in Era 2.
*Conclusions: SOT recipients treated in Era 2, when the major targeted therapies were remdesivir, dexamethasone, and convalescent plasma, were not at higher risk for renal dysfunction, ACR, or AMR in the aftermath of COVID-19; rejection was uncommon in both eras and mortality was low in both eras. While awaiting detailed safety studies, these results suggest against renal toxicity or triggering of alloimunity in those receiving newer therapies.
To cite this abstract in AMA style:
Chiang T, Sait A, Massie A, Marr K, Brennan D, Cochran W, Shah P, Jain T, Steinke SMehta, Desai N, Permpalung N, Shoham S, Merlo C, Durand C, Werbel W, Dioverti M, Ostrander D, Gurakar A, Kim MY, Garonzik-Wang J, Segev D, Avery R. Comparison of Outcomes in Sot Recipients During Two Eras of Covid-19 Therapeutics [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/comparison-of-outcomes-in-sot-recipients-during-two-eras-of-covid-19-therapeutics/. Accessed January 11, 2026.« Back to 2021 American Transplant Congress