Practice Patterns in Pneumocystis Jirovecii Pneumonia Prophylaxis in Solid Organ and Bone Marrow Transplant Recipients
S. Cao, K. Khalil, F. Cirrone, S. Mehta
NYU Langone Transplant Institute, New York, NY
Meeting: 2021 American Transplant Congress
Abstract number: 723
Keywords: Lung infection, Prophylaxis
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
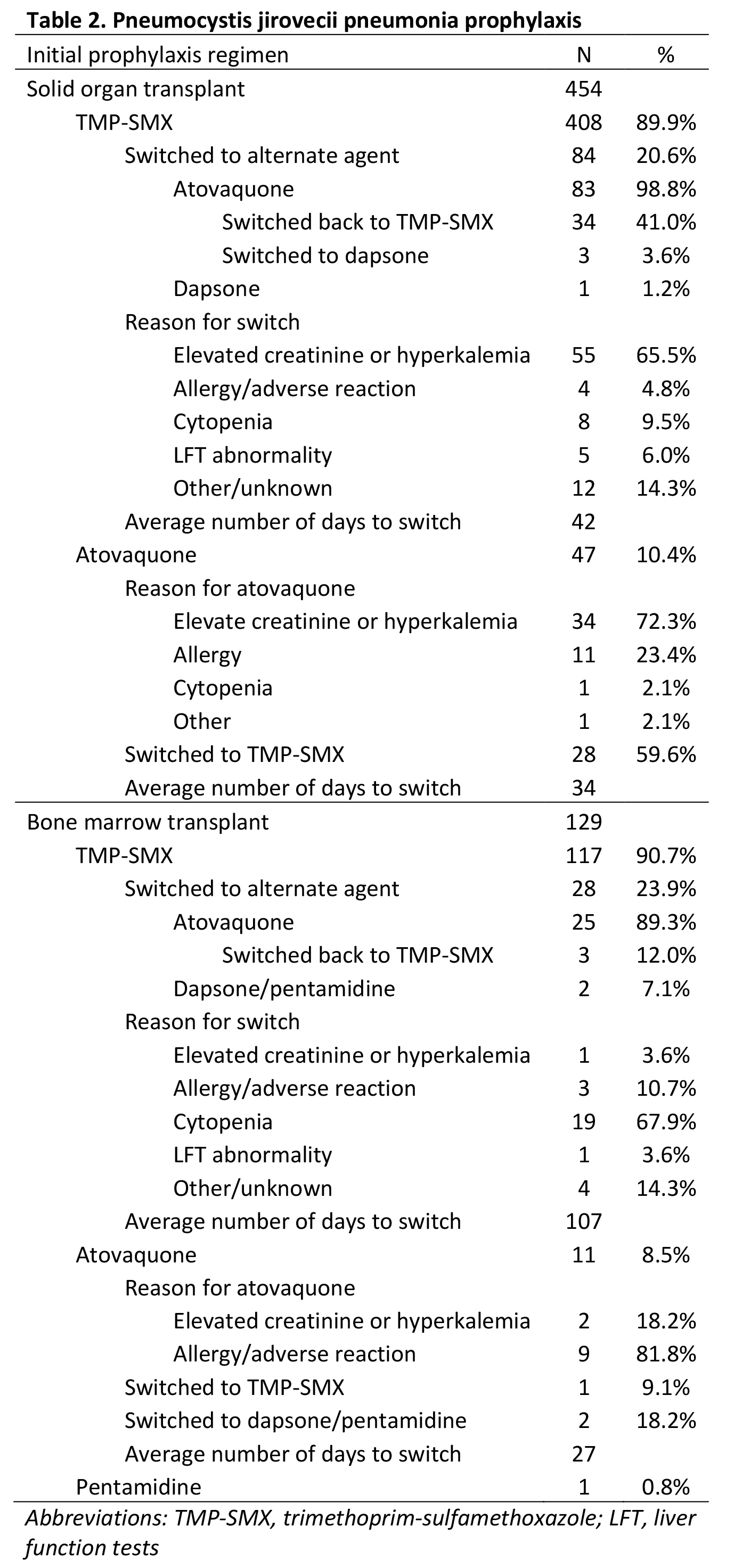
*Purpose: Trimethoprim-sulfamethoxazole (TMP-SMX) is the preferred agent for pneumocystis jirovecii pneumonia (PJP) prophylaxis in solid organ transplant (SOT) and bone marrow transplant (BMT); however, adverse effects may lead to the use of alternative agents. We sought to characterize the rate and reason for using alternative agents for PJP prophylaxis in SOT and BMT recipients over an 18-month period at a single institution to guide stewardship.
*Methods: SOT and BMT patients between 2/1/2018 and 8/1/2019 were included. 455 SOT and 126 BMT patients were identified and included in the final analysis; 1 SOT and 2 BMT patients were excluded due to expiration prior to initiation of PJP prophylaxis. Electronic medical records were retrospectively reviewed for initial PJP prophylaxis choice, change of initial prophylaxis, and reason for change.
*Results: Among 454 SOT and 124 BMT recipients, most (90.1%) patients received initial prophylaxis with TMP-SMX, followed by atovaquone (9.9%). In SOT recipients, the primary reason for an initial or subsequent switch to an alternative regimen was elevated creatinine or potassium. In BMT recipients, the primary reason was cytopenias. Among patients who were switched to an alternate regimen, 34 (41%) SOT recipients and 3 (12%) BMP recipients were switched back to TMP-SMX. Among patients who were initiated on an alternate regimen, 28 (59.6%) SOT recipients and 1 (9.1%) BMT recipient were switched to TMP-SMX. Mean number of days to TMP-SMX regimen interruption was 42 days in SOT recipients and 107 days in BMT recipients (p=0.005).
*Conclusions: While most SOT and BMT recipients received TMP-SMX for PJP prophylaxis, alternate agents were used primarily in the setting of concern for renal and hematologic adverse effects. Patients should be monitored for recovery of renal function and cell counts to evaluate the appropriateness of switching back to TMP-SMX.
To cite this abstract in AMA style:
Cao S, Khalil K, Cirrone F, Mehta S. Practice Patterns in Pneumocystis Jirovecii Pneumonia Prophylaxis in Solid Organ and Bone Marrow Transplant Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/practice-patterns-in-pneumocystis-jirovecii-pneumonia-prophylaxis-in-solid-organ-and-bone-marrow-transplant-recipients/. Accessed February 16, 2026.« Back to 2021 American Transplant Congress