One-year Outcomes of a Multicenter Trial of Transplantation of Hcv Viremic Kidney Donors into Hcv Uninfected Recipients
M. Sise1, D. Goldberg2, D. Schaubel3, J. Kort4, R. Alloway5, J. Friedewald6, R. Fontana7, S. Sultan8, N. Desai9, R. Chung1, P. Reese10
1Mass General Hospital, Boston, MA, 2U-Miami, Miami, FL, 3U. Pennsylvania, Philadelphia, PA, 4Abbvie, Chicago, IL, 5U Cincinnati, Cincinnati, OH, 6Northwestern U., Evanston, IL, 7U. Michigan, Ann Arbor, MI, 8Cornell, New York City, NY, 9Johns Hopkins, Baltimore, MD, 10U. Pennslyvania, Philadelpha, PA
Meeting: 2021 American Transplant Congress
Abstract number: LB 12
Keywords: Donors, marginal, Hepatitis C, Kidney
Topic: Clinical Science » Infectious Disease » Non-Organ Specific: Viral Hepatitis
Session Information
Session Name: Late Breaking: Basic & ID
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:15pm-6:20pm
Presentation Time: 6:15pm-6:20pm
Location: Virtual
*Purpose: As interest in transplantation of Hepatitis C virus (HCV)-viremic kidneys into HCV-uninfected (HCV+ to HCV– KT) recipients increases, understanding clinical outcomes beyond HCV clearance with direct-acting antivirals is important. We report the one-year outcomes of the seven-center MYTHIC (Multi-center studY to Transplant Hepatitis-C InfeCted Kidneys) trial.
*Methods: Donors were HCV RNA positive, had any HCV genotype, with KDPI < 85. The 30 KT recipients were treated with Glecaprevir-pibrentasvir (G/P) for 8 weeks, starting 2-5 days post-KT. We assessed the following 1 year post transplant: HCV virologic status, Cytomegaloviurs (CMV), and polyoma virus (BK) infection, transplant rejection, graft function, and patient survival. We performed a comparison of time-to-transplant with recipients of non-HCV infected kidneys derived from UNOS and matched based on a multivariate risk score.
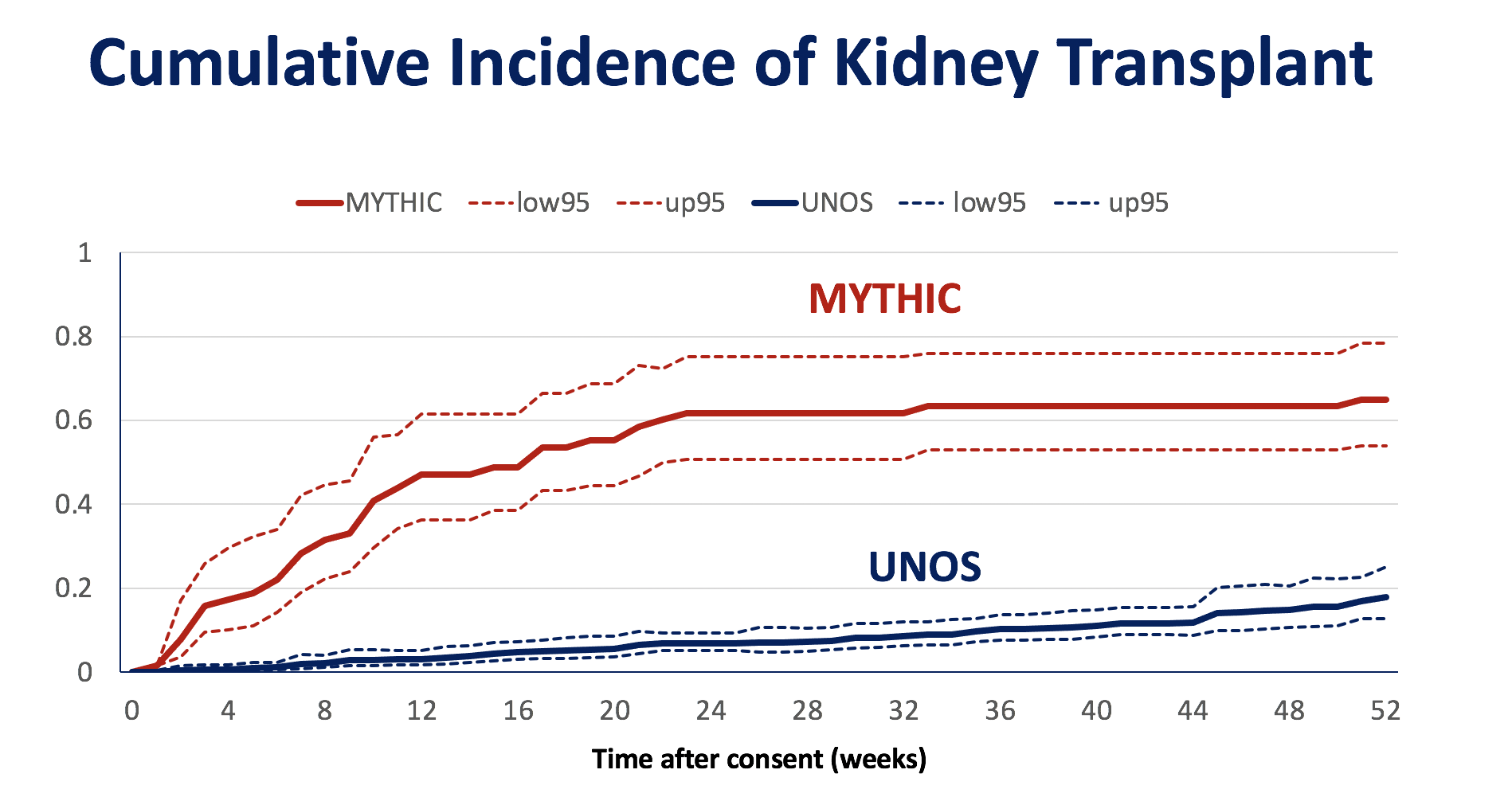
*Results: Seventy-six patients were consented for evaluation, 12 were excluded, and of 64 eligible patients, 30 underwent kidney transplant from HCV-viremic donors after a median of 6.3 weeks (IQR, 1.9-10.1). Patients enrolled in the MYTHIC trial were significantly more likely to receive a kidney transplant compared to risk-score matched UNOS comparators (N=642) (Figure). All 30 participants achieved durable HCV clearance and no patient developed clinically significant liver disease. There were 9 cases of detectable CMV viremia in the first year post-transplant, with 4 cases having > 1000 IU/mL. There were 4 cases of BK viremia > 1000 IU/mL. One year survival was 93%; there were two deaths after HCV cure (Staph aureus bacteremia and unexplained death at home). There were 3 cases of biopsy-confirmed acute rejection. One year graft function among the 28 surviving patients was excellent (mean serum creatinine 1.27mg/dL, SD 0.41).
*Conclusions: One year findings from the first multicenter standardized trial of pre-emptive G/P after HCV+ to HCV- KT demonstrate that this approach is highly effective, with excellent patient outcomes and shortened waitlist time to transplant.
To cite this abstract in AMA style:
Sise M, Goldberg D, Schaubel D, Kort J, Alloway R, Friedewald J, Fontana R, Sultan S, Desai N, Chung R, Reese P. One-year Outcomes of a Multicenter Trial of Transplantation of Hcv Viremic Kidney Donors into Hcv Uninfected Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/one-year-outcomes-of-a-multicenter-trial-of-transplantation-of-hcv-viremic-kidney-donors-into-hcv-uninfected-recipients/. Accessed February 18, 2026.« Back to 2021 American Transplant Congress