The Learning Curve Associated with De Novo Tacrolimus XR Use in a Racially Diverse Kidney Transplant Population
Medical University of South Carolina, Charleston, SC
Meeting: 2021 American Transplant Congress
Abstract number: 207
Keywords: African-American, FK506, Immunosuppression
Topic: Administrative » Quality Assurance Process Improvement & Regulatory Issues
Session Information
Session Name: Quality Assurance and Regulatory Issues
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 5:00pm-5:05pm
Presentation Time: 5:00pm-5:05pm
Location: Virtual
*Purpose: Tacrolimus XR (LCP-Tac) was approved for de novo dosing in kidney transplant recipients in 2018. In 2020, our center was challenged with a nationwide shortage of tacrolimus IR, warranting implementation of a new de novo LCP-Tac protocol. The purpose of this study is to assess LCP-Tac outcomes with 3 initial dosing strategies in kidney transplant recipients
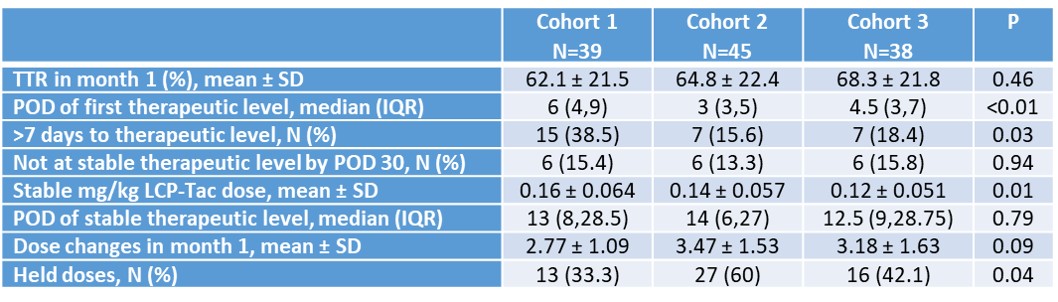
*Methods: This was a retrospective longitudinal study of adult kidney recipients transplanted between May and September 2020. The study population was divided into 3 cohorts based on sequential de novo LCP-Tac dosing strategies: 0.12 mg/kg in all patients (cohort 1), 0.17 mg/kg in all patients (cohort 2), and most recently, 0.12 mg/kg in non-African Americans and 0.15 mg/kg in African Americans. The primary endpoints were days to achieve therapeutic level and time in therapeutic range (TTR) at 1-month post-transplant. Categorical data were analyzed using chi square or fishers exact. Continuous data were analyzed using Kruskal-Wallis.
*Results: A total of 122 patients were included. Baseline characteristics were similar between cohorts. Cohorts 2 and 3 achieved a therapeutic level in roughly 3-4 days, compared to 6 days in cohort 1 (p<0.01) and 38% of cohort 1 did not achieve a therapeutic level by day 7 vs. 16-18% in cohorts 2 and 3 (p=0.03). TTR was slightly higher in cohort 3 (68%), as compared to cohorts 1 and 2 ((62-65%); p=0.46). There were more held doses in cohort 2 (60%). Other peri-operative outcomes were similar between cohorts, as were acute rejection rates and graft loss.
*Conclusions: Using a stratified de novo LCP-Tac dosing strategy based on African-American race appears to strike the best balance between achieving therapeutic levels quickly after transplant while preventing giving too high of an initial dose. Studies determining the impact of CYP3A5 genotyping are ongoing and may help further clarify this dosing strategy.
To cite this abstract in AMA style:
Bartlett F, Carcella T, Patel N, Rohan V, Taber D. The Learning Curve Associated with De Novo Tacrolimus XR Use in a Racially Diverse Kidney Transplant Population [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/the-learning-curve-associated-with-de-novo-tacrolimus-xr-use-in-a-racially-diverse-kidney-transplant-population/. Accessed March 6, 2026.« Back to 2021 American Transplant Congress