Comparable Outcomes for Simultaneous Liver-Kidney Transplantation with HCV NAT-Positive Donors
1Surgery, Duke University Hospital, Durham, NC, 2Medicine, Duke University Hospital, Durham, NC
Meeting: 2020 American Transplant Congress
Abstract number: D-011
Keywords: Allocation, Hepatitis C, Kidney, Outcome
Session Information
Session Name: Poster Session D: Kidney Living Donor: Other
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: To evaluate the impact of hepatitis C virus (HCV) Nucleic Acid Amplification-positive (NAT+) donors on outcomes following simultaneous liver-kidney (SLK) transplantation.
*Methods: We used the UNOS database to identify the cohort of adult patients receiving SLK 2015 (NAT testing was mandated) to 2018. Patients listed before and after the July 10, 2017 change in SLK listing policy were analyzed separately. Patient and death-censored graft survival were compared at 1 year using chi-squared and multivariable Cox proportional hazard models adjusted for recipient gender, race, KDRI, and transplant MELD.
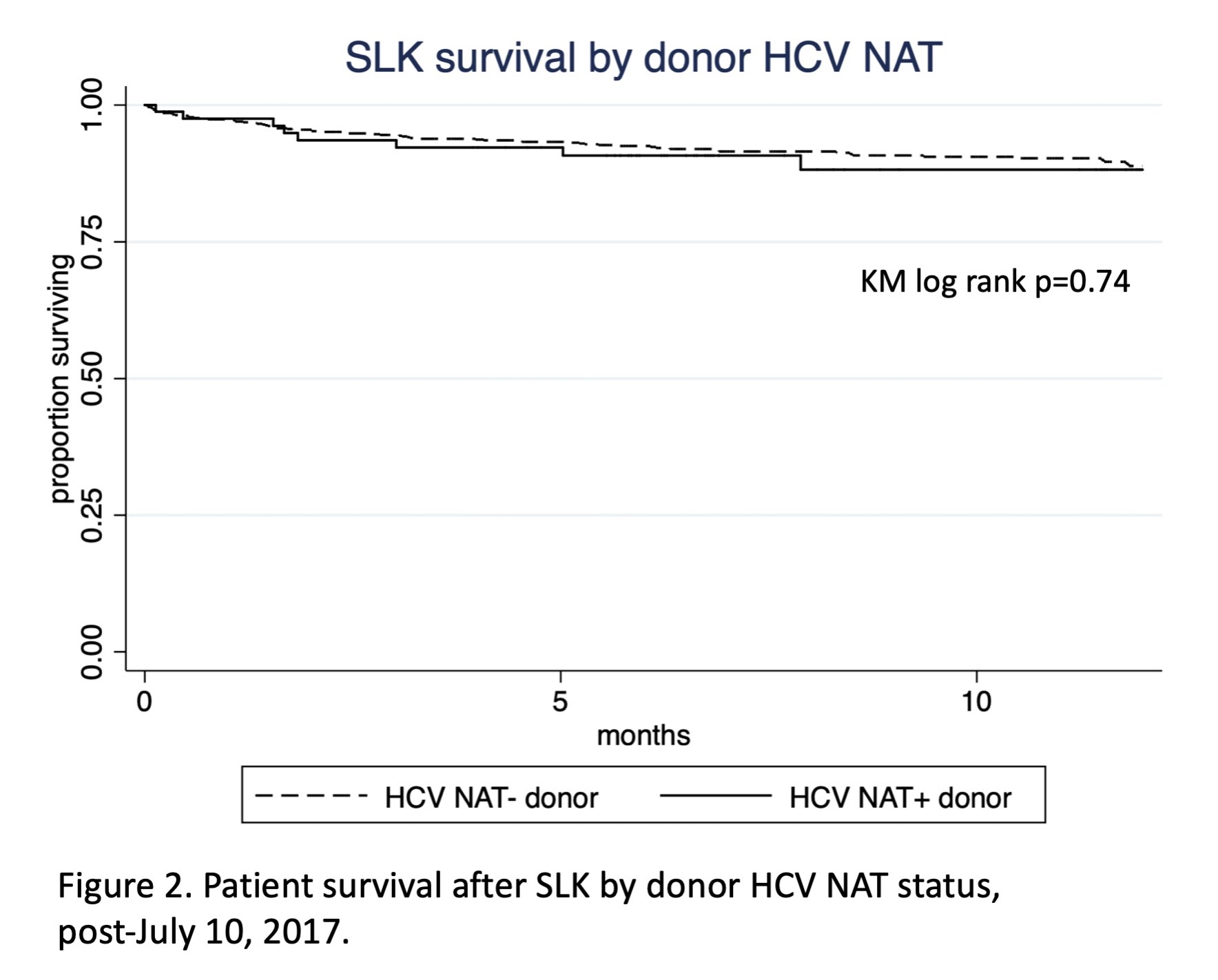
*Results: During this period, 2701 SLKs were performed in the US, 1790 before the SLK listing policy change and 911 after. In total, 227 (8.4%) patients received NAT+ donors, and 32 (14.5%) NAT+ grafts were used in HCV seronegative recipients (Figure 1). Compared to NAT- donors, NAT+ donors were of similar age, had higher KDPI (49% vs 34%, p<0.0005), and lower BMI (25 vs 26, p=0.001). Pre-policy, unadjusted 1-year patient survival trended higher in those receiving NAT+ than NAT-negative organs (94.5% vs 90.15%, p=0.088); 1-year liver graft survival was higher in NAT+ recipients (95.2% vs 89.8%, p=0.036). Post-policy, unadjusted 1-year survival was not affected by donor NAT status (NAT+90.2%, negative 91.3%, p=0.74), nor was graft survival (Figure 2). Post-policy risk-adjusted death did not differ by donor NAT status [HR 1.24 (95% CI 0.60-2.58, p=0.56)]. Patient and graft survival were similar for HCV seronegative vs. seropositive recipients of NAT+ organs after the policy change, though this comparison was limited by low sample size (n=26).
*Conclusions: The widespread adoption of NAT and direct acting antivirals have allowed the reliable identification of HCV-viremic donors at high risk of transmitting HCV. Under the current SLK policy, donor NAT+ does not significantly impact patient and graft survival, even among HCV seronegative recipients. However, given low sample size, further study is necessary to delineate the impact of acute HCV transmission on long-term outcomes of the especially vulnerable SLK population.
To cite this abstract in AMA style:
Samoylova ML, Shaw BI, Kesseli SJ, McElroy L, Patel Y, Sanoff S, Barbas AS. Comparable Outcomes for Simultaneous Liver-Kidney Transplantation with HCV NAT-Positive Donors [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/comparable-outcomes-for-simultaneous-liver-kidney-transplantation-with-hcv-nat-positive-donors/. Accessed February 26, 2026.« Back to 2020 American Transplant Congress