Real-World Experiences in the Transplantation of Hepatitis C Positive Kidneys
J. M. Steinbrink, J. Byrns, L. Crona, M. Ellis, S. Sanoff, E. K. Maziarz, C. R. Wolfe
Duke University, Durham, NC
Meeting: 2020 American Transplant Congress
Abstract number: B-197
Keywords: Hepatitis C, Kidney transplantation
Session Information
Session Name: Poster Session B: Non-Organ Specific: Viral Hepatitis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
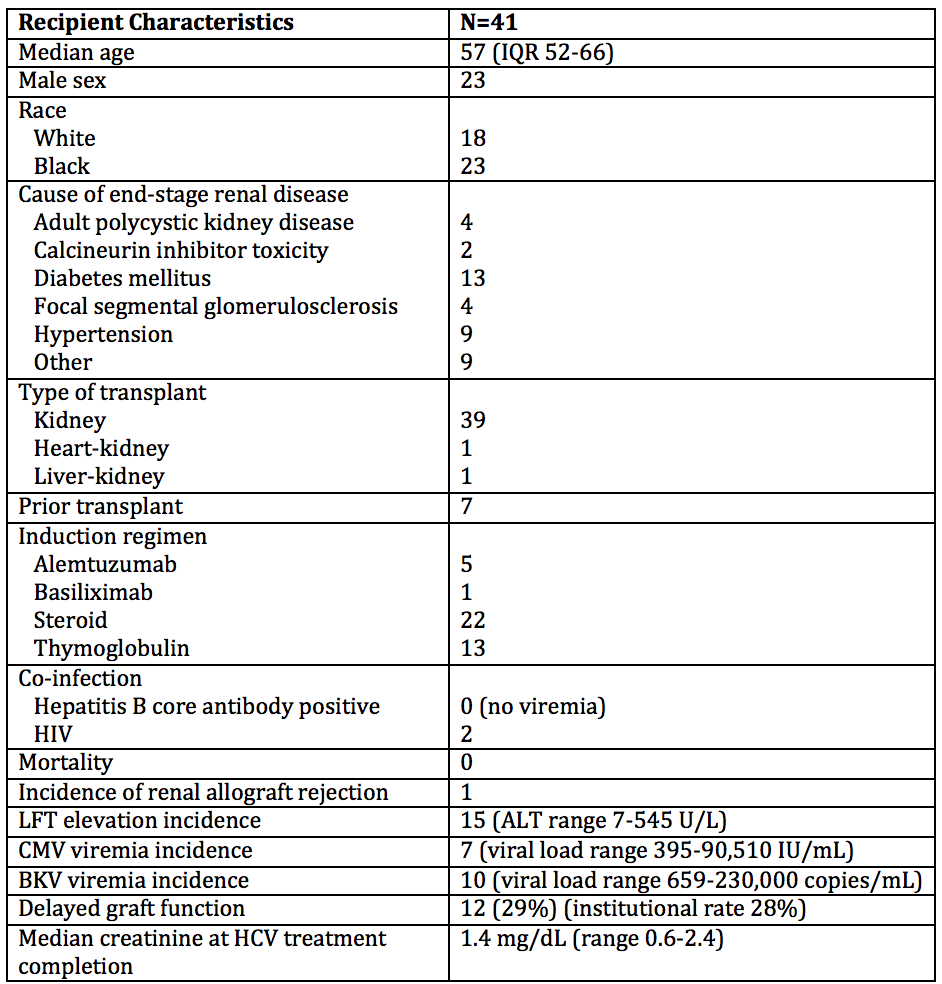
*Purpose: With advancements of direct-acting antivirals, transplantation of HCV-NAT+ (HCV+) organs to HCV-negative (HCV-) recipients has been used to decrease wait times without apparent sacrifice of patient safety or graft function. We present data from the first 41 renal transplant recipients at our center and describe our approach to outpatient post-transplant initiation of HCV therapy in a real-world setting.
*Methods: We performed a retrospective chart review of all HCV- recipients who received a renal transplant from a HCV+ donor from 12/2018-11/2019 at Duke University. Transplant data and clinical outcomes were reviewed.
*Results: Forty-one transplants were performed from HCV+ donors from 15 states; 40 recipients became viremic in the first week. Median peak viral load was 709,126 IU/mL. Genotype 3 was seen in 24% of recipients, higher than the reported national prevalence. Median time from listing to transplant was 537 days, yet from HCV+ consent to transplant was only 43 days; 5 patients were transplanted pre-dialysis.
All recipients received outpatient HCV treatment for 12 weeks, with a median time to treatment of 42 days post-transplant. Pan-genotypic HCV regimens were frequently used, most commonly glecaprevir/pibrentasvir. Liver function tests (LFT) were elevated in 37% of recipients. Only 10% exceeded 3x the upper limit of normal, with one transient peak of 545 IU/L. All transaminitis resolved with treatment. All patients who completed HCV treatment became aviremic, those followed to 12 weeks after therapy completion maintained a sustained virologic response.
There was 100% recipient survival, and delayed graft function rates were comparable to our institutional average. There was one episode of Banff 1a biopsy-proven rejection. CMV infection requiring treatment was seen in 17% of recipients, without end organ disease. We observed no extra-hepatic HCV effects or BK nephropathy.
*Conclusions: Recipient wait times to transplantation are significantly decreased when using organs from HCV+ donors. In a real-world setting, recipients from such donors develop rapid viremia, yet are able to achieve treatment cure in a timely manner, without apparent compromise of graft function or patient safety.
To cite this abstract in AMA style:
Steinbrink JM, Byrns J, Crona L, Ellis M, Sanoff S, Maziarz EK, Wolfe CR. Real-World Experiences in the Transplantation of Hepatitis C Positive Kidneys [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/real-world-experiences-in-the-transplantation-of-hepatitis-c-positive-kidneys/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress