Evaluation of the Use of Sublingual Tacrolimus in the Inpatient Setting
1Pharmacy, UNC Medical Center, Chapel Hill, NC, 2Surgery-Transplant, UNC Medical Center, Chapel Hill, NC
Meeting: 2020 American Transplant Congress
Abstract number: A-241
Keywords: Drug interaction, Immunosuppression, Monitoring, Safety
Session Information
Session Name: Poster Session A: Quality Assurance Process Improvement & Regulatory Issues
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: To determine the efficacy and safety of sublingual (SL) tacrolimus (TAC) administration.
*Methods: A single-center, retrospective study was performed on solid organ transplant recipients who received SL TAC between 6/1/2014 – 6/30/2018. Recipients were included if they were >18 years old, received at least four doses of oral TAC before converting to SL TAC, and received at least two consecutive days of SL TAC. Exclusion criteria included recipients receiving oral extended release formulations or intravenous TAC before conversion to SL TAC, and recipients without a baseline oral TAC trough concentration (TTC) before conversion to SL TAC. The primary endpoint was the mean change in TTC on oral TAC prior to conversion compared to SL TAC. Secondary endpoints included the amount of time recipients were subtherapeutic, therapeutic, and supratherapeutic, incidence of incorrect drug administration (within 15 minutes of SL TAC), and incidence of acute rejection and graft loss within six months post-conversion.
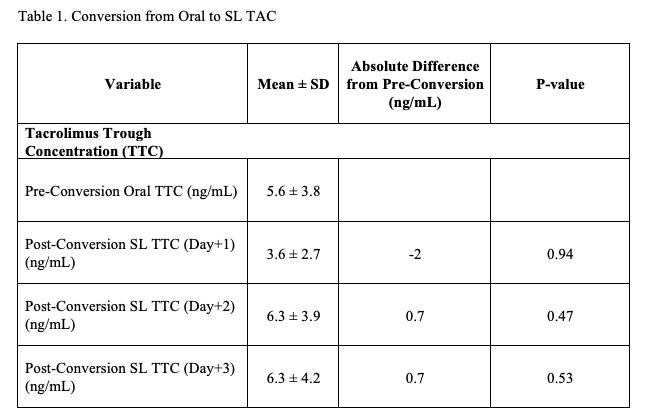
*Results: 135 solid organ transplant recipients received SL TAC, and 32 recipients met inclusion criteria. No significant differences in TTC were observed after conversion from oral to SL TAC (Table 1). Therapeutic TTC was achieved in 18.8% of recipients receiving oral TAC and in 25% of recipients after conversion to SL. The overall mean percentage of time within the therapeutic range during SL administration was 12.5% (±20.5) while 13.3% (±23.7) and 74.2% (±32.7) of the time was supratherapeutic and subtherapeutic, respectively. 62.5% of the time on SL TAC had incorrect drug administration. Acute rejection occurred in 34.4% of recipients with 27.3% of these within one month of conversion to SL TAC (median time 82.5 days (IQR 50.8-114.3)). No graft loss occurred.
*Conclusions: There was no significant difference in TTC in recipients converted to SL TAC. Due to the rate of incorrect drug administration, a separate SL TAC inpatient order was created with specific preformed administration instructions. An evaluation of the operational challenges along with increased medication safety initiatives aimed at correcting proper drug administration could improve efficacy and rate of therapeutic TTC.
To cite this abstract in AMA style:
Szempruch K, Kemp L, Barber A, Mason S, Offei-Nkansah G, Gerber D, Owen P. Evaluation of the Use of Sublingual Tacrolimus in the Inpatient Setting [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/evaluation-of-the-use-of-sublingual-tacrolimus-in-the-inpatient-setting/. Accessed February 5, 2026.« Back to 2020 American Transplant Congress