Cost-Effectiveness of Protocol Biopsies in Kidney Transplantation
Medicine, University of Pittsburgh, Pittsburgh, PA
Meeting: 2020 American Transplant Congress
Abstract number: 617
Keywords: Economics, Protocol biopsy, Rejection
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes VI
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
*Purpose: To perform an economic evaluation of protocol biopsies in kidney transplantation
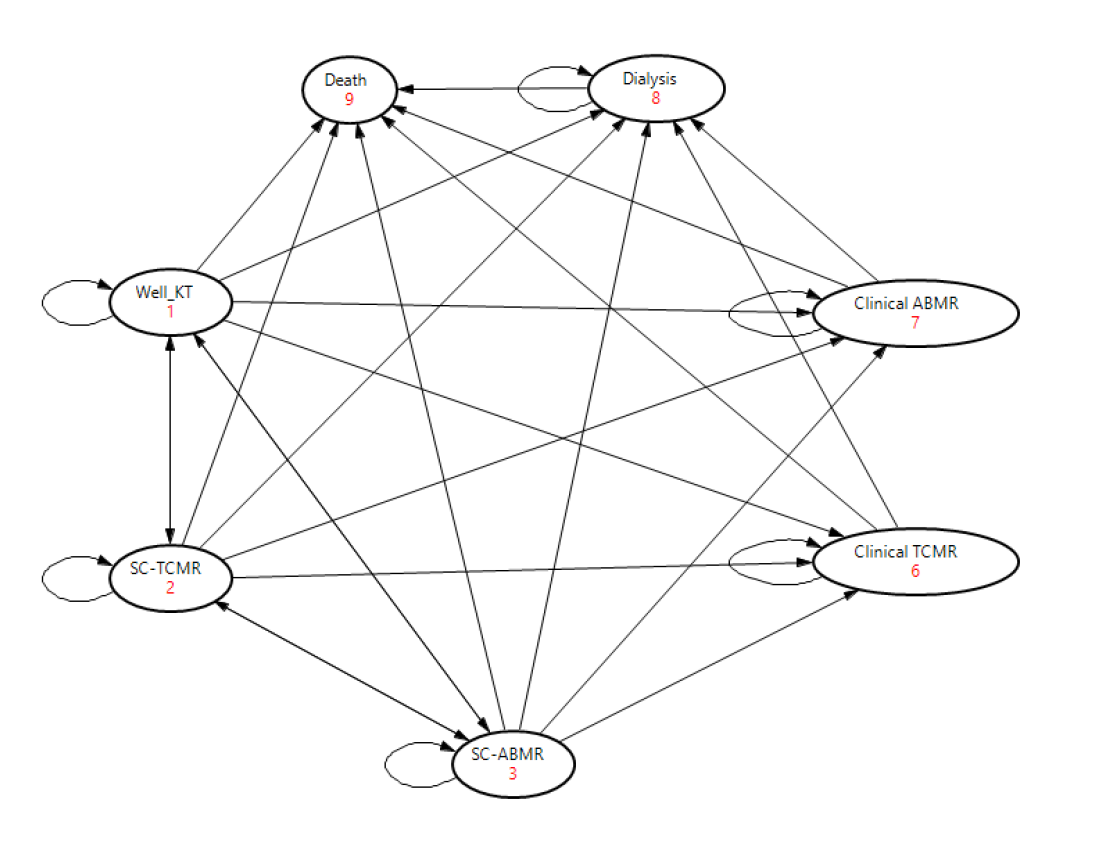
*Methods: We used a Markov decision analytical model (Figure 1) to compare the following strategies for detecting sub-clinical rejection (SCR) in the first year after kidney transplantation (KT): no protocol biopsy (PB), PB at 3 months, PB at 12 months, PB at 3 and 12 months and PB at 3, 6 and 12 months. Costs, utilities and probabilities were derived from published reports (select base case values are shown in the table). Key model inputs and assumptions were: 1) Both sub-clinical T-cell mediated rejection (SC-TCMR) and sub-clinical antibody mediated rejection (SC-ABMR) were included 2) All SCR episodes were treated 3) 50% of SCR occurred between 0 and 3 months and 50% occurred between 3 and 12 months after KT. 4) Biopsy had 100% sensitivity and specificity for detecting SCR. A lifetime analysis was performed. Incremental cost effectiveness ratios (ICERs) were calculated for each strategy, and one-way sensitivity analysis were performed.
*Results: On base case analysis, ICER for 12-month PB over no PB was $37,660 per quality adjusted life year (QALY) gained. 3-month PB yielded lower QALYs than 12-month PB at higher cost. QALY gains from increasing PB frequency during first year were small and resulted in high ICERs ($186,862 for PB at 3 and 12 months, and $272,978 for PB at 3,6 and 12 months). Table 3 shows the results of one-way sensitivity analysis of PB at 12 months over a no PB strategy. Results were most sensitive to patient age, and relative risk (RR) for graft failure from SC-TCMR and SC-ABMR. Cost-effectiveness worsened with increasing patient age and lower RR for graft failure from SC-TCMR and SC-ABMR. Results were also sensitive to biopsy cost, prevalence of SCR, and SCR treatment efficacy.
*Conclusions: In kidney transplantation, protocol biopsy more than once during the first year is not economically reasonable. Cost-effectiveness varied significantly with age, biopsy cost, SCR treatment efficacy, and SCR impact on graft survival.
| Variable | Base case value | >$50,000 per QALY | >$100,000 per QALY |
| Incidence of SC-TCMR at year 1 (%) | 12 | <7 | — |
| Relative Risk of graft loss due to untreated SC-TCMR | 1.3 | <1.25 | <1.1 |
| Incidence of SC-ABMR at year 1 (%) | 3 | <2 | — |
| Relative Risk of graft loss due to untreated SC-ABMR | 3 | <2.2 | <1.3 |
| Probability of SC-TCMR treatment failure | 0.3 | >0.5 | >0.8 |
| Probability of SC-ABMR treatment failure | 0.5 | >0.65 | >0.8 |
| Cost of kidney transplant biopsy (US $) | 1500 | >1900 | >3600 |
To cite this abstract in AMA style:
Puttarajappa CM, Hariharan S, Smith KJ. Cost-Effectiveness of Protocol Biopsies in Kidney Transplantation [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/cost-effectiveness-of-protocol-biopsies-in-kidney-transplantation/. Accessed February 25, 2026.« Back to 2020 American Transplant Congress