Feasibilty of Hepatitis C NAT Positive Kidney Transplant into Hepatitis C Negative Recipients as Standard of Care
University of Cincinnati Medical Center, Cincinnati, OH
Meeting: 2020 American Transplant Congress
Abstract number: 340
Keywords: Cadaveric organs, Hepatitis C, Kidney transplantation, Outcome
Session Information
Session Name: Kidney Deceased Donor Selection II
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:27pm-4:39pm
Presentation Time: 4:27pm-4:39pm
Location: Virtual
*Purpose: With advent of direct acting antivirals (DAA) for Hepatitis (Hep) C which have a 96-100% sustained viral remission(SVR), doors have opened for using Hep C nucleic acid amplification testing (NAT)+ kidneys and treat the patients for acquired Hep C post-transplant. We performed a prospective IRB approved trial at our center to validate the use and challenges of this approach.
*Methods: Hep C NAT negative patients on the kidney transplant wait list were approached and informed consent was obtained. Treatment was planned post transplant after approval was obtained from their insurance company. In addition, our center agreed to cover the treatment cost if denied by insurance company. Patients with Hep C related kidney disease, chronic liver disease, dual organ transplants, HIV and Hep B infection were excluded. Post-transplant, they were tested for Hep C viral load and genotype starting day 3, 7 and weekly thereafter until Hep C viremia was confirmed. DAA was prescribed by hepatologist according to the Hep C genotype and insurance company preference. Standard of care immunosuppression protocols and post-transplant monitoring was done.
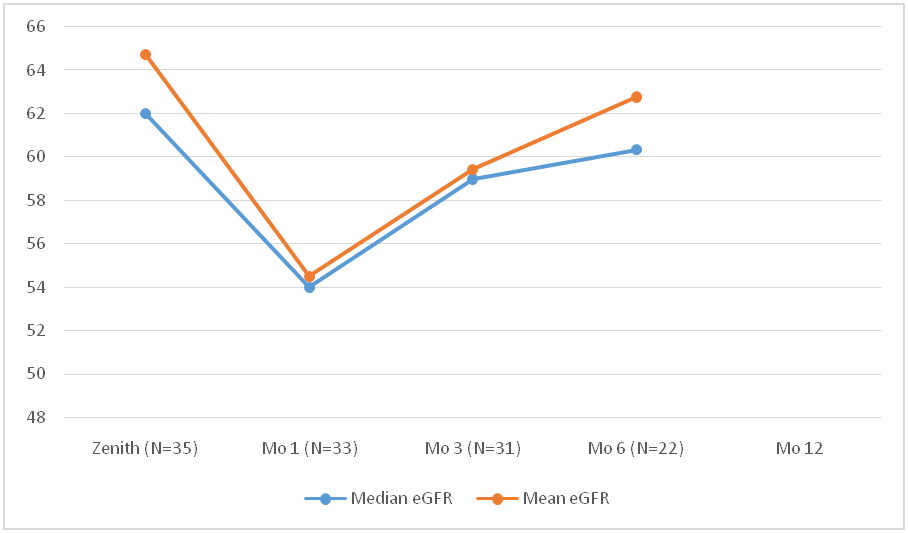
*Results: 35 patients received Hep C NAT+ kidneys between 10/2018- 10/2019 (80% males, 20% females). Median age of the recipients was 53 years (range 29-72). Blood group distribution was 18(51%), 14(40%), 2(6%) and 1(3%) patients for O, A, B and AB blood group respectively. Mean waiting time for recipients was 820 ± 654 days. Mean KDPI was 56 ±15 with a median donor age of 38 years (range 22-56). All patients became viremic between day 7-10. 57 % (20) had genotype 1a, 28% (10) had 3 and 14 %( 5) had 2b. 5 pts had transient transaminitis (>2X AST/ALT). All patients got their Hep C treatment approved through their insurance. Median time from transplant to DAA initiation day was 43 days (range 15-94). As of Nov. 2019, 33/35 (94%) patients have been treated and all (100%) have become Hep C RNA negative (end of treatment response EOT) with 16 patients (48%) achieving 12-week SVR. The remaining 2 patients are yet to start the therapy. The kidney function has remained stable during and after the course of treatment (fig.1). 1 patient had glomerulonephritis recurrence and 1 patient had tubuloreticular inclusions on allograft biopsy attributed to Hep C infection.
*Conclusions: Transplantation of Hep C NAT positive kidneys to Hep C negative recipient followed by treatment of Hep C is a feasible option as a standard of care outside the sponsored trials. Recipient should be closely monitored for Hep C related kidney complications.
To cite this abstract in AMA style:
Kaur T, Choi J, Anwar N, Anand M, Shah S, Bumb S, Jawdeh BAbu, Shah S, Diwan T, Govil A. Feasibilty of Hepatitis C NAT Positive Kidney Transplant into Hepatitis C Negative Recipients as Standard of Care [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/feasibilty-of-hepatitis-c-nat-positive-kidney-transplant-into-hepatitis-c-negative-recipients-as-standard-of-care/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress