The Impact of Purposeful Reductions in Immunosuppression on Clinical Outcomes in Kidney Transplantation
K. Foster, H. Perkins, N. Patel, C. Perez, N. Pilch, H. Meadows, F. Bartlett, V. Rao, M. Casey, M. Posadas Salas, K. Soliman, J. McGillicuddy, D. Taber
MUSC Health, Charleston, SC
Meeting: 2020 American Transplant Congress
Abstract number: 272
Keywords: Immunosuppression, Infection, Kidney transplantation, Outcome
Session Information
Session Name: Kidney Infections Excluding Polyoma & Viral Hepatitis
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: Reductions to immunosuppressive therapy (IS) are often made due to infection or other IS intolerances, but little is known of impact of these reductions on graft outcomes. This study aimed to assess impact of IS reductions on clinical outcomes in kidney transplant recipients.
*Methods: This was a single-center, retrospective, longitudinal cohort study that evaluated changes in IS in the first-year post-transplant in kidney transplant recipients from 1/2017-2/2019. Exclusion criteria were pediatric recipients, dual organ recipients, patients who transferred care, <1 year follow up, patients with primary outcome within first year, identical twin HLA matched transplants, and use of experimental IS. Patients initially received tacrolimus (FK), mycophenolate mofetil (MMF), and prednisone as maintenance IS. Baseline and clinical follow-up assessments were acquired via electronic and manual chart abstraction. Groups included no IS reduction, reduced IS for infection, or reduced IS for noninfectious reasons (e.g. leukopenia, diarrhea). Reduced IS was defined as average MMF daily dose <1400 mg or average monthly FK level <6 for >2 months in the first year post-transplant. Outcomes were included if the first event occurred >1-year post-transplant. The primary outcome was composite graft loss, death, eGFR<30 mL/min/1.73 m2, Banff grade >1A, treated borderline rejection, and biopsy-proven moderate to severe fibrosis after 1 year. Pearson Chi Square and Cox Regression were used to analyze results.
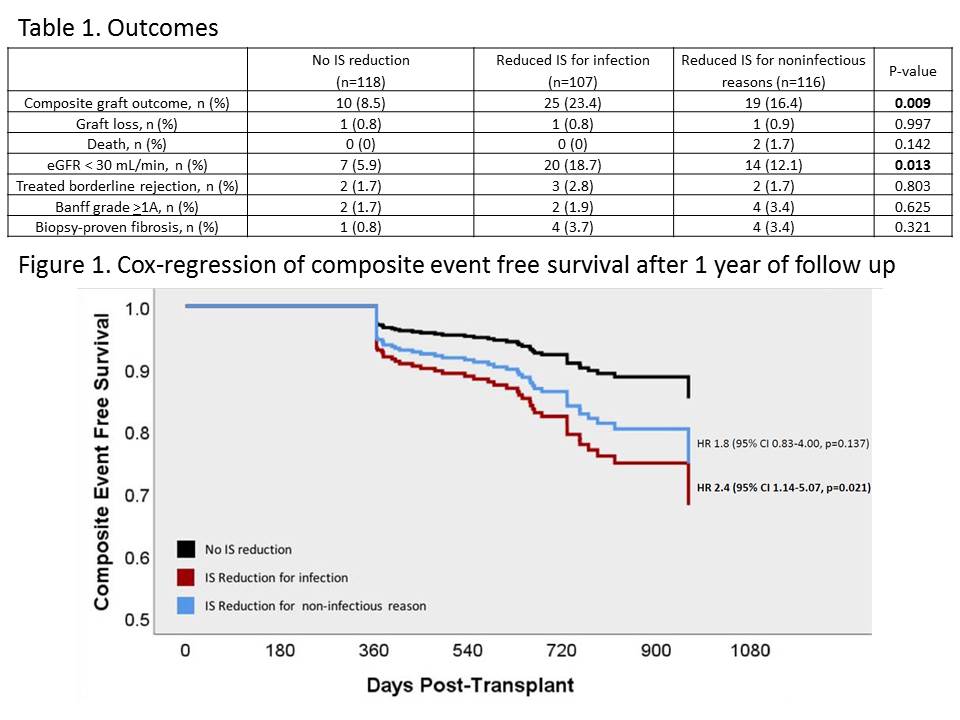
*Results: Of 564 patients assessed, 341 met criteria and were analyzed. Baseline characteristics were similar between groups; though patients with no IS reduction had a significantly lower age and KDPI. Compared to no IS reduction, patients with reduced IS for infection had a lower composite event free survival (aHR 2.4, 95% CI 1.14-5.07, p=0.021; Figure 1). The major differences in outcomes were seen in graft function; patients with reduced IS for infection were more likely to have an eGFR <30 mL/min/1.73 m2 (Table 1).
*Conclusions: Reduction in IS for infection <1-year post-transplant significantly increases the risk of deleterious events after one-year post-transplant. While reduced IS may promote resolution of infection, providers should recognize the potential risks of prolonged IS reduction and assess for appropriateness to return to standard IS dosing.
To cite this abstract in AMA style:
Foster K, Perkins H, Patel N, Perez C, Pilch N, Meadows H, Bartlett F, Rao V, Casey M, Salas MPosadas, Soliman K, McGillicuddy J, Taber D. The Impact of Purposeful Reductions in Immunosuppression on Clinical Outcomes in Kidney Transplantation [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/the-impact-of-purposeful-reductions-in-immunosuppression-on-clinical-outcomes-in-kidney-transplantation/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress