Use of Sitagliptin versus Placebo to Reduce the Incidence and Severity of Post-Transplant Diabetes after Kidney Transplantation
H. Wijeweera, J. Hagopian, M. Ramakrishnan, L. Chen, R. Delos Santos
Washington University in St. Louis, Saint Louis, MO
Meeting: 2020 American Transplant Congress
Abstract number: 125
Keywords: Kidney transplantation, Metabolic complications, Post-transplant diabetes
Session Information
Session Name: Kidney: Cardiovascular and Metabolic Complications I
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Virtual
*Purpose: Post-transplant diabetes mellitus (PTDM) is a complication in non-diabetic kidney transplant (KT) recipients due weight gain and use of CNI and corticosteroids in immunosuppression regimens. Non-diabetic KT recipients with early postoperative hyperglycemia have higher rates of PTDM compared to normoglycemic patients. We hypothesized that temporary use of sitagliptin (SIT) could be utilized in hyperglycemic KT recipients for prevention of PTDM.
*Methods: We performed a single-center, double-blind, randomized-controlled trial to evaluate the efficacy of SIT for the prevention of PTDM in KT recipients with hyperglycemia (BS>200mg/dL) within the first 5 days post-KT. Patients were excluded if they had a history of diabetes (DM), baseline A1c >6.5%, or received a dual-organ or kidney after a non-renal solid organ transplant. Patients received SIT or placebo (PLB) for 3 mo, performed an OGTT test, then discontinued SIT or PLB. At 6 mo patients had a second OGTT test. SIT or PLB was dosed per renal function post-KT. If needed, insulin or oral non-DPP4-I therapies were added. The efficacy assessment at 3 mo was 3-fold: a difference in 2-hour OGTT BG by ≥20mg/dL, attainment of a normal 2-hour OGTT BG, and improvement in A1c by ≥0.5%. Continuous variables were compared using two-sample t-test or Mann-Whitney U test and categorical variables were compared using Chi-square test or Fisher Exact test as appropriate. All statistical tests are two-sided at significance level 0.05.
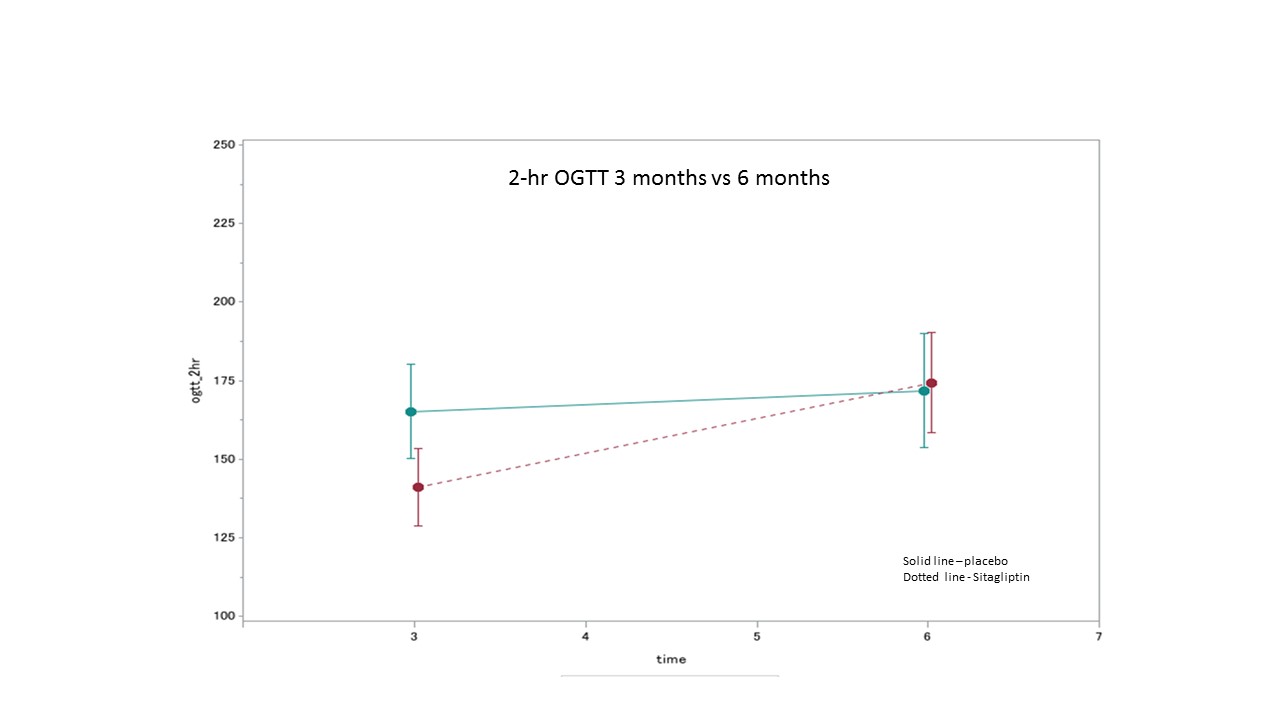
*Results: In total, 61 patients were enrolled and 50 patients were included in the modified ITT analysis. There were no differences noted in baseline characteristics including age, sex, race, cause of ESRD, transplant type, comorbidities, or family history of DM. The mean 2-hour OGTT BG results in the SIT and PLB arms at 3 mo was 165.2mg/dL and 141.0mg/dL, respectively. This met the goal difference in the efficacy assessment of ≥20mg/dL but this difference was not significant (p=0.22). In the study arm the mean 2-hour OGTT BG of 141mg/dL neared but did not reach the goal attainment of a normal 2-hour OGTT BG of <140mg/dL. The A1c in the SIT and PLB arms at 3 mo was 5.78% and 5.53%, respectively. The difference in A1c of 0.25% was not significant (p=0.25), and did not meet the goal efficacy endpoint of ≥0.5%. There were no safety concerns that required discontinuation of SIT in a preventative setting.
*Conclusions: SIT did not show statistical significance in the 3-fold efficacy assessment, but can be considered to improve 2-hour OGTT results and glycemic control to near normal in the early post-KT setting.
To cite this abstract in AMA style:
Wijeweera H, Hagopian J, Ramakrishnan M, Chen L, Santos RDelos. Use of Sitagliptin versus Placebo to Reduce the Incidence and Severity of Post-Transplant Diabetes after Kidney Transplantation [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/use-of-sitagliptin-versus-placebo-to-reduce-the-incidence-and-severity-of-post-transplant-diabetes-after-kidney-transplantation/. Accessed February 8, 2026.« Back to 2020 American Transplant Congress